Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(5); 2022 > Article
-
Review ArticleHypothalamus and Pituitary Gland Independent Skeletal Actions of Pituitary Hormones
 Keypoint
Keypoint
The pituitary gland, often called the “master gland,” orchestrates multiple effector hormonal organs and other glands by secreting various tropic hormones. Over the past years, pituitary hormones and their receptors have been shown to have non-traditional actions that allow them to bypass the hypothalamus-pituitary-effector glands axis. In this review, the authors discuss the interactions of each pituitary hormone with bone and the potential of these interactions for understanding bone physiology and as therapeutic targets. -
Se-Min Kim
 , Farhath Sultana, Funda Korkmaz, Daria Lizneva, Tony Yuen, Mone Zaidi
, Farhath Sultana, Funda Korkmaz, Daria Lizneva, Tony Yuen, Mone Zaidi
-
Endocrinology and Metabolism 2022;37(5):719-731.
DOI: https://doi.org/10.3803/EnM.2022.1573
Published online: September 28, 2022
The Mount Sinai Bone Program, Departments of Pharmacological Sciences and Medicine, and Center of Translational Medicine and Pharmacology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- Corresponding authors: Se-Min Kim. The Mount Sinai Bone Program, Departments of Pharmacological Sciences and Medicine, and Center of Translational Medicine and Pharmacology, Icahn School of Medicine at Mount Sinai, PO Box 1055, New York, NY 10029, USA Tel: +1-212-241-8797, Fax: +1-212-426-8312 E-mail: se-min.kim@mountsinai.org
- Mone Zaidi. The Mount Sinai Bone Program, Departments of Pharmacological Sciences and Medicine, and Center of Translational Medicine and Pharmacology, Icahn School of Medicine at Mount Sinai, PO Box 1055, New York, NY 10029, USA Tel: +1-212-241-8797, Fax: +1-212-426-8312, E-mail: mone.zaidi@mssm.edu
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- INTRODUCTION
- UBIQUITOUS EXPRESSION OF PITUITARY HORMONE RECEPTORS
- HIGH FSH AND BONE LOSS
- TSH AND BONE: NOT JUST THYROXINE
- ADRENOCORTICOTROPIC HORMONE AND BONE
- THE MAIN PLAYER: GH OR IGF1
- PROLACTIN AND BONE MASS
- OXYTOCIN AND INTERGENERATIONAL CALCIUM TRANSFER
- VASOPRESSIN AND BONE
- CONCLUSIONS
- Article information
- References
ABSTRACT
- Over the past years, pituitary hormones and their receptors have been shown to have non-traditional actions that allow them to bypass the hypothalamus-pituitary-effector glands axis. Bone cells—osteoblasts and osteoclasts—express receptors for growth hormone, follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH), adrenocorticotrophic hormone (ACTH), prolactin, oxytocin, and vasopressin. Independent skeletal actions of pituitary hormones on bone have been studied using genetically modified mice with haploinsufficiency and by activating or inactivating the receptors pharmacologically, without altering systemic effector hormone levels. On another front, the discovery of a TSH variant (TSH-βv) in immune cells in the bone marrow and skeletal action of FSHβ through tumor necrosis factor α provides new insights underscoring the integrated physiology of bone-immune-endocrine axis. Here we discuss the interaction of each pituitary hormone with bone and the potential it holds in understanding bone physiology and as a therapeutic target.
- The pituitary gland, often called the “master gland” orchestrates multiple effector hormonal organs and other glands by secreting various tropic hormones, namely growth hormone (GH), follicle stimulating hormone (FSH), luteinizing hormone (LH), thyroid stimulating hormone (TSH), adrenocorticotrophic hormone (ACTH), prolactin, oxytocin (OXT), and vasopressin. It is well-known that effector hormones, particularly thyroid hormone, cortisol, insulin-like growth factor 1 (IGF1), and sex hormone (i.e., estrogen and testosterone) play a significant role in skeletal modeling and remodeling. We have learned over the past decade that pituitary hormones also directly exert skeletal actions. The reciprocal relationship with pituitary and effector hormones poses a significant challenge in differentiating direct from indirect actions of pituitary hormone, but sophisticated genetic modification and small molecule intervention without altering systemic levels of effector hormone levels has allowed us to examine the direct skeletal role of these glycoproteins. These non-traditional skeletal effects of pituitary hormones will be discussed in this review.
INTRODUCTION
- The hypothalamic-pituitary-endocrine (i.e., adrenal, thyroid, and ovaries/testis) feedback axis is truly fascinating physiology that establishes the central concept of endocrinology. The physiology of endocrine system and pathological changes from the imbalance—hormonal excess or insufficiency and their skeletal complication are well studied.
- Over the past years, it has become increasingly clear that the pituitary tropic hormones also exert non-traditional actions bypassing the endocrine axis. Many studies have now documented the ubiquitous expression of the G-protein coupled receptors (GPCRs) for pituitary hormones, establishing that these receptors are not solely localized to endocrine organs. For example, in mammals, the TSH receptors (TSHRs) are expressed in the pituitary gland, thymus, testis, kidney, brain, adipose tissue, bone and heart albeit at lower levels [1,2]. The genetically modified mice with replacement of exon 1 of the TSHR with green fluorescent protein (GFP) visualized non-thyroidal expression of TSHR [3,4]. TSHR expression has been documented previously in osteoblast-like rat osteosarcoma cells (UMR106 cells), rodent calvaria-derived primary osteoblasts, pre-osteoblastic MC3T3-E1 cells, human osteoblast-like cells, osteoclast precursors, and mature osteoclasts [2,5-9].
- Likewise, apart from their expression in granulosa cells of the ovary and Sertoli cells of the testis [10-12], FSH receptors (FSHRs) are present on bone, fat and the nervous system [4,13-15]. In the human female reproductive tract and placenta, FSHR is expressed in vascular endothelial cells, endometrial glands, cervical glands and stroma, stromal cells and muscle fiber, and interstitial macrophage from testis [16,17]. Furthermore, in endothelial cells of various cancer tissues (i.e., breast, colon, pancreas, kidney etc.), FSHR expression was much higher than gonadotropin cells [18]. Also, chondrocyte-like ATDC5 cells expressed functional FSHRs [19]. Interestingly, our data using RNAscope showed FSHR expressions in cerebral cortex and hippocampus, particularly in granular layer and pyramidal tract [13]—this suggested a role for FSH-FSHR interactions in cognition. An FSHR type 2 isoform without exon 9 is found in human monocytes and osteoclasts at a lower levels compared with ovarian cells. Of note, FSHR has 10 exons with the first 9 exons encoding extracellular domain and exon 10 expressing transmembrane domain [20-22].
- ACTH receptor (MC2R), one of five melanocortin receptors (MC1R–MC5R), is expressed predominantly in the adrenal glands and required for adrenal gland development and steroidogenesis [23], is also expressed in bone cells—osteoblast-like cells (MG63), SaOS2 cells, and normal human osteoblasts (NHOS). ACTH and other proopiomelanocortin (POMC)-derived peptides increased cyclic adenosine monophosphate (cAMP) levels in NHOS [24]. Interestingly, immune cells, namely macrophages, splenic B and T-cytotoxic (CTL) cells were capable of secreting ACTH [25], and osteoclasts, which are differentiated from hematopoietic stem cells, not only expressed and processed POMC and secreted ACTH, but also expressed MC2R [24], suggesting possible autocrine or paracrine actions of ACTH in bone remodeling.
- Other pituitary hormones also have their receptors expressed in bone and bone cells. The findings of prolactin receptors on osteoblasts in cell culture and bone tissue suggested possible direct effect of prolactin in bone remodeling independently of concomitant hypogonadism in the setting of hyperprolactinemia [26-28]. OXT and vasopressin, which are secreted from posterior pituitary gland, also have their receptors expressed in osteoblasts and osteoclasts [29]. The functional relevance of the pituitary hormone receptor expression in skeletal tissue and bone cells will be reviewed in the following section.
UBIQUITOUS EXPRESSION OF PITUITARY HORMONE RECEPTORS
- FSH was first isolated from sheep pituitary gland and FSH injection increased the size of ovarian follicles in hypophysectomized rats suggesting its reproductive role [30]. Likewise, after receiving partially purified FSH preparation, amenorrheic women showed increased urinary estrogen levels and increased the size of the uterine cavity and ovaries [31]. Subsequently, it was shown that FSH-containing cells are present in the pituitary gland. A negative feedback loop between FSH and estradiol was subsequently documented [32-35].
- Our group first described an effect of FSH in skeletal remodeling using genetically modified FSHR and FSHβ mice. Haploinsufficient mice for both the ligand and receptor had normal estrogen levels and developed an intact uterus—yet they showed higher bone mass compared with wild-type mice, essentially separating the action of FSH from that of estrogen [36]. Knock-out mice were also protected, but in Fshr−/− mice, this protection due to absent FSHR signaling was confounded by the associated hyperandrogenemia [37]. A recent study performed µCT analysis of distal femurs from male and female Fshb+/+, Fshb+/−, and Fshb−/− mice at 8 months of age and showed higher bone volume and less trabecular spacing in the absence of Fshβ [38].
- Using interventional approaches, lowering FSH in the ovariectomized rat by gonadotropin-releasing hormone (GnRH) agonist (leuprolide) decreased the number of osteoclasts and bone loss area in alveolar bone [39]. Likewise, blocking the FSH effect using anti-FSHβ antibodies decreased osteoclast differentiation up to approximately 20% [40]. More importantly, blocking with anti-FSHβ antibody attenuated ovariectomy-induced bone loss [4,14,15,40]. Furthermore, we found that the skeletal effect of FSH was independent of other hormones, including estrogen, testosterone, inhibin, or activin. This finding was consistent with a recent study where humanized monoclonal anti-FSHβ antibody (MS-Hu6) treatment reduced osteoclast formation, and did not alter LH, GnRH, or testosterone levels [41]. Complementing these data, it was shown that an FSH-glutathione-S-transferase (GST) fusion protein prevented ovariectomy-induced bone loss in rats [42].
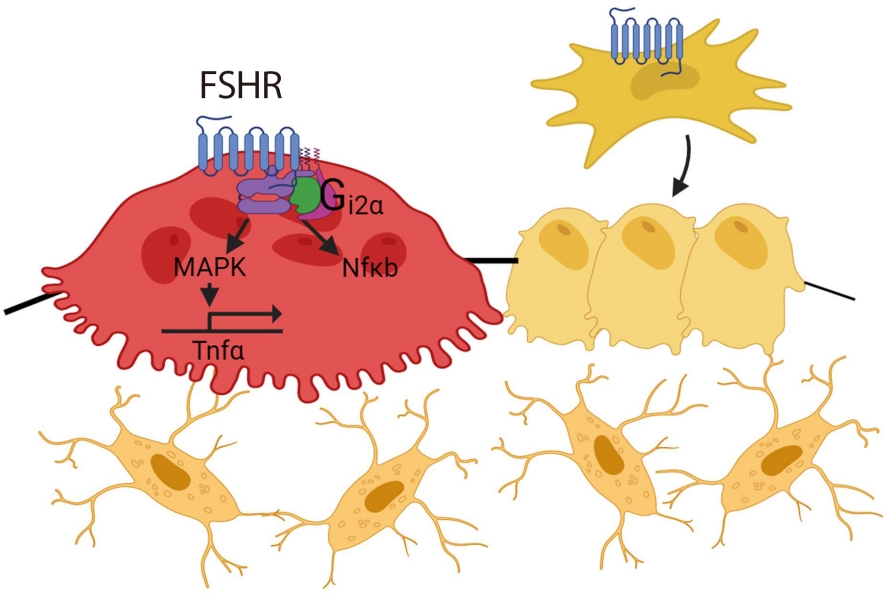
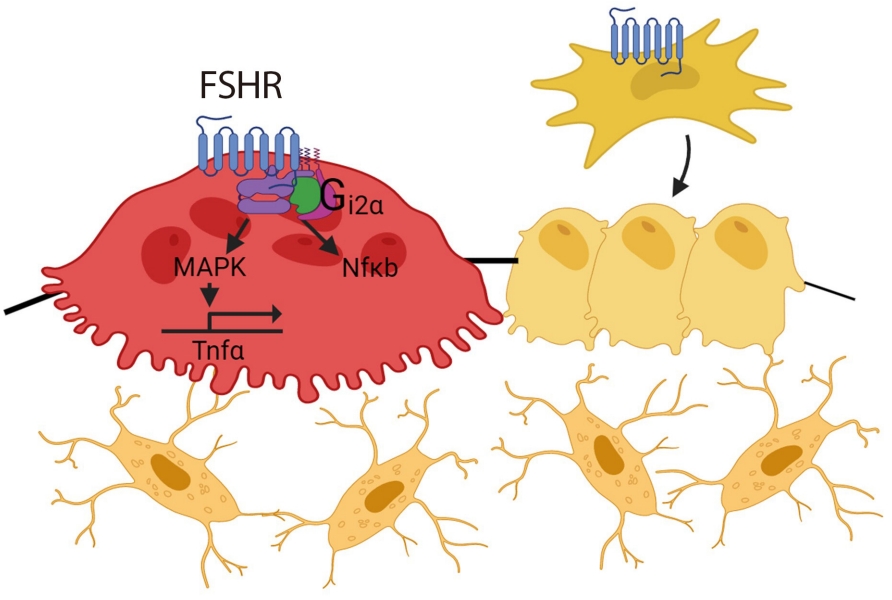
- FSH acts on the bone FSHR isoform that is coupled to the G-protein Gi2α, and in doing so, increases osteoclastogenesis and bone resorption and suppresses bone formation [20,36,40,43]. FSH also enhances RANK expression and indirectly promotes osteoclastogenesis by stimulating the release (or enhancing the receptor expression) of tumor necrosis factor α (TNFα), interleukin (IL)-1β, and IL-6 [20,21,44-46]. FSH-induced osteoclast differentiation is also abolished in bone marrow macrophages from mice lacking immunoreceptor tyrosine-based activation motif (ITAM) adapters (Fig. 1) [47].
- FSH negatively regulates osteoblastic bone formation. FSHRs are expressed on mesenchymal stem cells (not mature osteoblasts), and FSH antibody treatment yields greater osteoblast precursor colony counts similar to mesenchymal cells isolated from Fshr null mice [20,43]. MS-Hu6 antibody treatment also showed increased bone formation with increased mineralization apposition rate (MAR) and up-regulated osteogenic genes (Alpl, Col1a1, and Runx2) [41].
- Supporting the mouse studies, multiple observational studies have examined associations between bone parameters and serum FSH. Notably, bone mineral density (BMD) of lumbar spine (LS), femur, and forearm show a negative correlation with an increase in serum FSH level, although these findings were limited due to the reciprocal relationship of FSH and E2 (High FSH and low E2) [48,49]. Other studies have attempted to examine the association after controlling for the estrogen effect. A study that sub-categorized the peri- and post-menopausal healthy women with irregular menstruation by FSH levels showed that participants with high FSH (>40 mIU/mL) had lower BMD compared with subjects with lower FSH levels (<40 mIU/mL) [50]. FSH increase by 1 standard deviation (SD) was associated with a 5% decrement in LS BMD in pre-, peri-, and post-menopausal women when E2 levels were similar in pre- and peri-menopausal women, but 2-fold higher FSH levels in the latter [51]. Bone turnover markers (BTMs) (i.e., urinary deoxypyridinoline [D-Pyr], urine N-terminal telopeptide [NTx], urinary C-terminal telopeptide [CTX], serum osteocalcin, and bone alkaline phosphatase) were shown to correlate with FSH positively, suggesting high bone turnover with elevated FSH levels [51-53].
- Comparing skeletal changes in patients with hypothalamic functional amenorrhea versus hypergonadotropic amenorrhea provided another line of evidence for a direct FSH effect. In patients with premature ovarian failure who were younger than the age of 40, those with hypergonadotropic amenorrhea (FSH >40 IU/L) had lower LS BMD than patients with hypogonadotropic amenorrhea despite having other risk factors for low BMD, such as nutritional deficit and underweight [54].
- The most relevant clinical correlations between elevated FSH and bone loss were noted in the Study of Women’s Health Across the Nations (SWAN), a longitudinal cohort of 2,375 perimenopausal women with diverse ethnic backgrounds. There was a strong positive correlation with serum FSH and NTx and osteocalcin independently of estrogen levels, and FSH was a stronger predictive marker for rapid bone turnover during perimenopausal period than estrogen [55]. Other large cohorts from China and Iceland (AGES-Reykjavik Study of Older Adults) also noted an inverse correlation between FSH and BMD [55-57]. In addition, the National Health and Nutrition Examination Survey (NHANES) III cohort showed a strong negative correlation between serum FSH and femoral neck (FN) BMD in perimenopausal women. In post-menopausal women, an incremental increase in FSH was associated with almost three-fold higher risk for osteoporosis, although the investigators used body mass index as a surrogate marker for estrogen status [58]. The bone turnover range of normality (BONTURNO) study similarly showed that women with high FSH levels (>30 IU/L) had high BTMs despite having regular menstruation [59]. Moreover, women with the activating FSHR polymorphism (rs6166) displayed lower bone mass and high resorption markers [60]. All of findings above suggest that the FSH surge during perimenopausal period might itself drive high bone turnover and bone loss. The selective inhibition of FSH action using antibodies such as ours may allow a further delineation of the effects of high FSH versus low estradiol.
HIGH FSH AND BONE LOSS
- Multiple observational studies have documented an association between low TSH levels and a low bone mass. Again, the reciprocal relationship between TSH and thyroid hormone (T4/T3) poses a challenge in understanding the independent skeletal effect of TSH. Most studies therefore used healthy euthyroid or subclinical hyperthyroid subjects.
- Analysis of the Rotterdam study showed a positive correlation between serum TSH levels and FN BMD among euthyroid subjects [61]. NHANES (1999 to 2002) showed similar results where low-normal TSH levels (0.39 to 1.79 mIU/L) were associated with a higher risk of osteoporosis independently of thyroid hormone levels [62]. Studies from China and Korea also noted similar patterns of associations [63-65].
- Associations of TSH levels with fracture risk is more challenging as fracture itself is a multi-factorial process with many confounding factors. The Osteoporosis and Ultrasound Study (OPUS) study showed a lower risk of fracture with higher TSH levels among euthyroid subjects [66]. However, other large epidemiology studies, namely the Tromsø study and the Rotterdam study did not find a clear correlation between TSH levels and fracture risk [61,67]. Lastly, a meta-analysis including thirteen studies of euthyroid adults showed an almost 1.25 times higher risk of hip fracture in a group with lower TSH levels (0.45 to 0.99 mIU/L) compared with subjects with high-normal TSH (3.50 to 4.49 mIU/L) [68].
- The negative skeletal effect of low TSH becomes more prominent when examining subjects with subclinical hyperthyroidism. The Study of Osteoporotic Fracture (SOF) showed that increased risk of fracture with a lower titer of TSH; participants with serum TSH (≤0.1 mIU/L) had a three-fold higher risk of hip fracture compared with normal TSH levels (0.5 to 5.5 mIU/L) [69]. The Odense Patient data Explorative Network Thyroid Status and Register Outcomes (OPENTHYRO) registry cohort also showed that low TSH levels (<0.3 mIU/L) was associated with a higher risk of hip fracture with 45% increase per 1 SD decrease of TSH [70]. Two separate meta-analyses summarizing 13 and six studies, respectively, again showed the hip fracture risk was significantly higher and BMD was lower in subclinical hyperthyroidism [71,72]. In addition, several studies suggested that micro-skeletal structure was suboptimal in elderly patients with subclinical hyperthyroidism and in patients under TSH suppression treatment following thyroidectomy for thyroid cancer [73-75]. However, this correlation of TSH and bone mass was not as robust in subclinical hypothyroidism, pre-menopausal women or men as in post-menopausal women [76].
- Several studies have also documented changes in BTMs after recombinant human TSH (rhTSH) injection. CTX and NTX levels dropped within days and procollagen type 1 N-terminal propeptide (P1NP), a marker of bone formation increased without changes in thyroid homorne levels [77-79].
- These human correlations point to a direct effect of TSH on bone, which was first described by us using TSHR haploinsufficient (Tshr+/–) mice. They displayed significant bone loss, despite intact thyroid follicles and normal thyroid hormone levels [2]. Homozygotic Tshr–/– mice were runted and had decreased BMD, which persisted despite thyroid hormone replacement from the birth [2,80]. The key characteristics of skeletal changes were summarized as increased bone turnover with predominantly increased osteoclast-driven bone resorption with focal sclerosis reminiscent of Paget’s bone disease [2]. In a later study, iatrogenic hyperthyroidism was induced by T4 pellet implantation after pre-treatment with carbimazole. Greater bone turnover and bone loss followed in Tshr–/– hyperthyroid mice compared with hyperthyroid wild-type, suggesting that the absence of TSH signaling contributed further bone loss in the setting of hyperthyroidism [81]. The skeletal phenotype of other genetically modified mice Tshrhyt/hyt with a loss-of-function mutation in TSHR (TSHRP556L) and Pax8–/–mouse, which lacks a transcription factor for thyroid follicle development were summarized in the recent reviews [76,82].
- The skeletal effect of TSH arises from a direct action on osteoclast TSHRs, but also indirectly through TNFα [80,83], an osteoclastogenic cytokine that is known to cause bone loss [45]. Immune-mediated TSH action on skeleton was demonstrated by interventional studies using TNFα antibody and genetic modification. Mice with TSHR deficiency showed increased osteoclastogenesis with up-regulated TNFα expression, but without any change in receptor activator of NF-κB-ligand (RANKL) or macrophage-colony-stimulating factor (M-CSF), two important signals for osteoclastogenesis [2]. Moreover, treatment with an anti-TNFα neutralizing antibody or crossing Tshr–/– mice with Tnfa–/– mice reversed increased osteoclast differentiation [2,80,83]. These findings are relevant to clinical observations, where patients with hyperthyroidism are accompanied by elevated TNFα and soluble TNF receptor (TNFR) levels [84]. Mechanistically, TSH inhibits high-mobility group box proteins (HMGB) 1 and 2 expression and their binding to DNA, and suppresses JNK1/2 and IĸBα phosphorylation and c-jun and p65 nuclear translocation, all of which are critical steps in TNFα synthesis [2,85]. Likewise, TSHR overexpression decreases the activator protein (AP)-1 and nuclear factor κB (NFκB) binding in response to RANKL and TNFα/IL-1 [83].
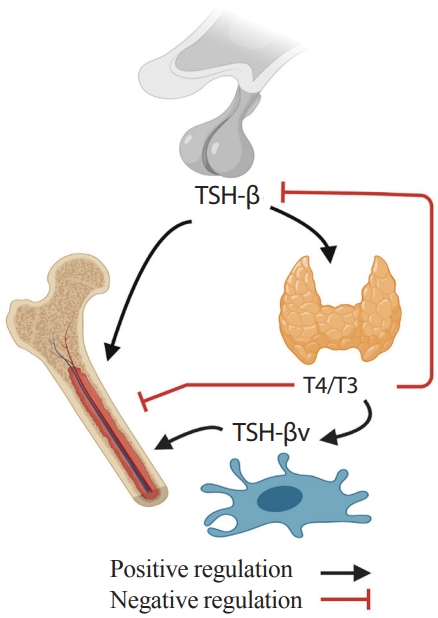
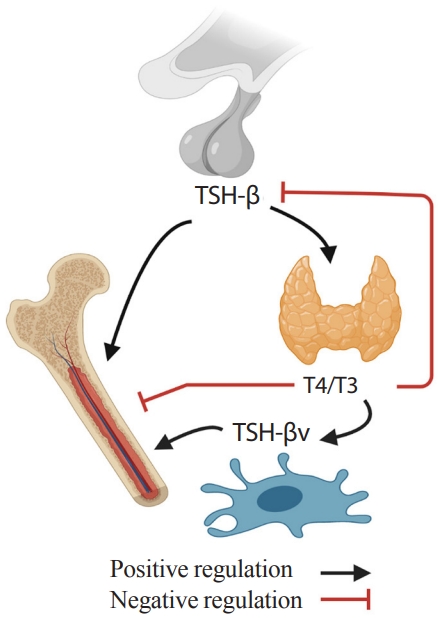
- An immune-skeletal-endocrine interaction of TSH is further supported by the identification of a splice variant of TSH (TSH-βv) in bone marrow cells [86,87]. Bone marrow-derived murine and human macrophages, and CD11b+ and other immune cells were shown to express TSH-βv [87,88]. This finding, in part, explains how TSHR in skeletal tissue can be functional despite being expressed at low levels [7,8].
- Interestingly, the regulation of TSH-βv does not follow traditional hypothalamic-pituitary-thyroid axis feedback. TSH-βv injection into mice increases serum T4/T3 levels, but TSH-βv expression in leukocytes was not increased or suppressed in response to thyrotropin releasing hormone (TRH) or T3, respectively [89]. Also, T4/T3 regulated TSH-βv expression positively at mRNA and protein levels rather than suppressing it [87,90]. In addition, pro-inflammatory cytokines suppressed pituitary TSH secretion [91], but at the same time, trafficked immune cells expressing TSH-βv to the thyroid glands [92]. These findings indicate that immune cells such as macrophage in bone marrow might have the potential to attenuate the negative skeletal effects of low TSH and high T4/T3 in pro-inflammatory hyperthyroidism by secreting TSH-βv. This proposed TSH-related circuitry involving the bone-immune-endocrine axis needs further study (Fig. 2).
- Unlike the anti-osteoclastic effect, TSH effects on osteoblasts are not as straightforward. In bone marrow-derived primary osteoblast cultures, TSH suppressed colony forming units and osteoblast gene expression, which was associated with downregulated vascular endothelial growth factor (VEGF) receptor FLK-1 and the Wnt co-receptor LRP5 [2]. However, other studies using calvaria-derived osteoblast, UMR106 and SaOS2 cell lines, show that TSH can stimulate osteoblastogenesis [6,93] through the activation of protein kinase Cδ and upregulation of the noncanonical Wnt components frizzled and Wnt5a [94]. In addition, co-culture of macrophages and osteoblasts enhanced osteoblastogenesis, which was attenuated in the presence of anti-TSH-βv antibody—this reaffirmed local skeletal effect of TSH-βv [87]. In vivo, intermittent low dose rhTSH injections induced bone formation and bone gain and recovered bone loss after ovariectomy [9]. Whether the anabolic action of TSH is dependent on the dose or the frequency similarly to parathyroid hormone needs to be further studied.
- Furthermore, TSHR might interact with IGF1 receptor (IGF1R). TSH up-regulated IGF1 and IGF2 expression as well as stimulatory IGF-binding proteins [93]. TSHR and IGF1R synergistically increase osteopontin through crosstalk between the receptors in a signaling complex through β-arrestin 1, scaffolds linking G-protein-coupled receptors to extracellular signal-regulated kinases 1/2 (Erk1/2) signaling [95]. TSH induces β-arrestin-1 binding to TSHRs, β-arrestin then phosphorylates Akt1, p38, and Erk1/2 and upregulates Alp, Rankl, and Opn. In contrast, downregulation of β-arrestin 1 inhibits TSH-mediated osteogenic gene upregulation [93,95,96]. In all, it seems clear at least experimentally—with observational evidence from large epidemiologic cohorts—that the net bone loss in hyperthyroidism is the result of interplay between components of the TSH-skeletal-immune axis, mediated by selective actions of T4/T3, TSH, and TSH-βv.
TSH AND BONE: NOT JUST THYROXINE
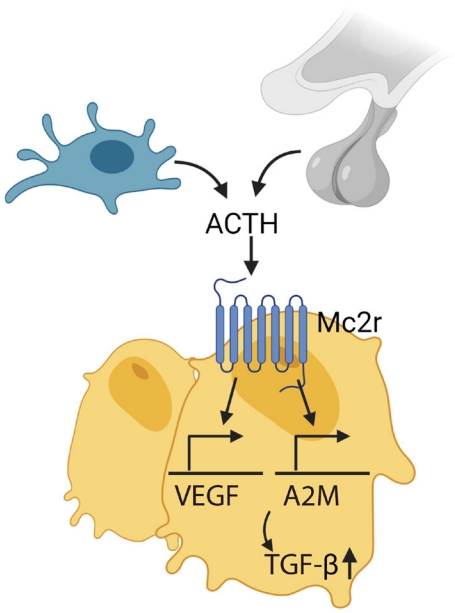
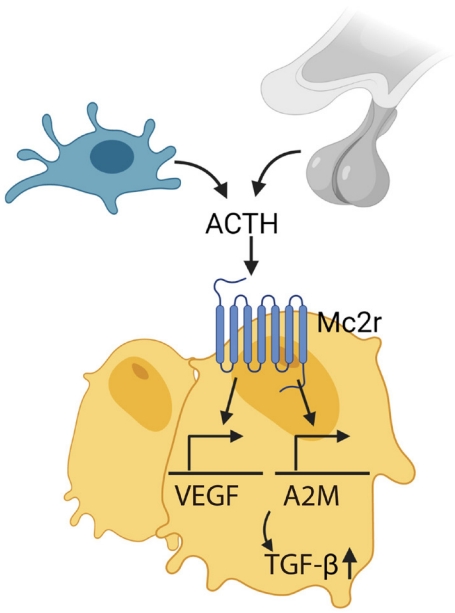
- ACTH directly effects bone bypassing glucocorticoid action. Our group showed that ACTH treatment in rabbit with methylprednisolone-induced avascular osteonecrosis of femoral head (AVFN) reduced necrotic surface, which likely was mediated by upregulating VEGF expression [97]. VEGF upregulation by ACTH was also shown in a later study [98]. In addition, ACTH enhances protease inhibitor alpha-2-macroglobulin (A2M), which likely promotes osteoblastic differentiation through transforming growth factor β induction (Fig. 3) [99]. These findings suggest that ACTH can be a therapeutic target for treating AVFN, which is often caused by vascular insufficiency from trauma, alcohol and more importantly, long-term steroid use [99,100]. However, patients with ACTH-dependent Cushing disease had less bone loss compared with those with ACTH-independent adrenal Cushing’s syndrome [101].
- A later study using Mc2r−/− mice showed increased cortical bone thickness without a change in trabecular bone. Serum osteocalcin levels were high in the homozygotes, while urinary deoxypyridinoline (D-Pyr) was decreased. However, unfortunately, the global knock-outs were confounded with adrenal insufficiency with deficiencies in glucocorticoid, mineralocorticoid, and catecholamines [102]. The skeletal phenotype of bone cell specific MC2R deficient mice has not been reported yet.
ADRENOCORTICOTROPIC HORMONE AND BONE
- While GH directly acts on bone through a GPCR, its action predominantly occurs through IGF1 [103]. IGF1 is synthesized mainly in the liver and approximately 80% circulates bound to IGF-binding protein-3 (IGFBP3) and the acid labile subunit (ALS). Studies using GH, GH receptor (GHR) and IGF1 deficient mice provided evidence of independent and common effects of GH versus IGF1 on the skeleton. The double Ghr/Igf1 knock-out mouse showed more severe growth retardation compared with either GHR deficient or IGF1 deficient mice [104]. Studies using liver-specific IGF1 and bone-specific IGF1 mouse delineated the systemic and local effects of IGF1. Unlike complete IGF1 knock-out mice, liver-specific IGF1-deficient mice, despite significant drop in circulating IGF1 (~75%), developed and grew normally, except displaying suboptimal cortical bone quality [105,106]. However, further lowering systemic IGF1 level below ~10% in liver-specific IGF deficient mice by deleting IGFBP3 and the ALS resulted in marked growth retardation [107,108]—this suggests that certain levels of systemic IGF1 are required. On the other hand, bone-specific IGF1 deficient mice with normal circulating IGF1 level showed significant growth retardation, low bone mass, impaired bone formation and mineralization [109]. Together, GH, systemic IGF1, and local IGF1 play concerted roles in postnatal skeletal growth, modeling and remodeling.
THE MAIN PLAYER: GH OR IGF1
- Prolactin receptor deficient (Prlr–/–) mice, generated by deleting exon 5 of the Prlr gene showed lower BMD in both sexes. Dynamic histomorphometry showed decreased bone formation. Of note, estrogen levels in female Prlr–/– mice were significantly lower compared with wild-type littermates, but testosterone in males did not differ. These findings can thus partly be explained by hypogonadism, but interestingly osteoclast surfaces, a hallmark of hypogonadal bone loss, were not different in Prlr–/– mice compared using wild-type littermates [28]. In contrast, a gain-of-function study in which hyperprolactinemia was induced by anterior pituitary transplantation in the setting of ovariectomy showed that prolactin stimulated bone turnover with net bone resorption. It was thought to be through decreased osteoprotegerin (OPG) expression in osteoblasts, which increased RANKL/RANK and osteoclastogenesis [27].
- Given its physiological role in lactation and procreation, the effect of maternal prolactin in embryonic skeletal development was examined. A study measured alkaline phosphatase as an indirect marker for bone turnover after prolactin administration during pregnancy. Newborn pups from treated dams showed almost ~30% decrease in alkaline phosphatase and reduced bone formation, without any change in calcium or parathyroid hormone levels [26]. This in vivo finding was consistent with in vitro osteoblast cell cultures using primary osteoblast and MG63 cells, where Alp and Ocn were downregulated and alkaline phosphatase activity was suppressed after prolactin treatment [26,27]. Whether prolactin exerts a protective bone effect by downregulating bone turnover in the setting of excessive maternal bone resorption needs to be further studied [110,111].
PROLACTIN AND BONE MASS
- OXT is a nonapeptide that is synthesized in hypothalamus and released via the posterior pituitary gland. OXT mediates milk ejection and uterine contraction during parturition, and regulates social behavior centrally [112,113]—but we have shown that it plays a role in calcium homeostasis and bone remodeling. Functional OXT receptors (OXTRs) are found in bone cells in both human osteoblasts [114] and osteoclasts [115].
- Oxt–/– and Oxtr–/– male and female mice displayed reduced bone mass with decreased bone formation. OXT treatment in cell culture stimulated osteoblast differentiation by upregulating Bmp2, Schnurri-2 and -3, osterix, and Atf-4 [116]. It also stimulated osteoclast formation by activating NFκB and mitogen-activated protein kinase (MAPK) signaling and indirectly through RANKL. Osteoclast-driven bone resorption was counteracted through cytosolic Ca2+ releases and nitric oxide (NO) synthesis, resulting in net anabolic effect [116]. In line with the findings, OXT decreased RANKL levels and increased OPG levels resulting in reduction in RANKL/OPG levels favoring net bone formation [117]. Osteoblast-specific Oxt–/–mice showed low bone mass, and osteoclast-specific Oxt–/– developed high bone mass, again confirming a stimulatory effect of OXT on osteoblastic bone formation and on osteoclast differentiation [118].
- We believe that OXT might play a role in skeletal mobilization through increased osteoclast formation during pregnancy and lactation [119]. Oxt–/– pups showed hypomineralized skeleton and Oxt–/– mom displayed reduced bone formation markers [119]. However, interestingly, pregnant, and lactating mice lacking OXTRs in osteoblasts showed higher bone mass, suggesting elevated OXT inhibits bone resorption to keep a balance against excessive bone loss during pregnancy and lactation (Fig. 4) [118].
OXYTOCIN AND INTERGENERATIONAL CALCIUM TRANSFER
- Arginine vasopressin (AVP), a key regulator of serum osmolality and fluid status, has also been implicated in bone remodeling. AVPR-1a and -2a are expressed in the osteoblasts and osteoclasts [120]. Vasopressin-deficient (AVPR-1a) mice displayed high bone mass due to increased bone formation and reduced bone resorption [29,120]. In addition, AVP administration to mice reduced osteoblast formation and increased osteoclast formation. On the other hand, AVPR-1a antagonist (SR49059) increased bone mass by promoting osteoblastogenesis and inhibiting osteoclast-driven bone resorption, together suggesting that vasopressin negatively regulates skeletal remodeling [120]. In contrast, AVPR-2a does not seem to have any skeletal effect as AVPR-2a inhibitor tolvaptan did not show any skeletal phenotype [29]. Our finding might explain the bone loss and high risk of fracture in patients with chronic hyponatremia that is often accompanied by high vasopressin levels.
VASOPRESSIN AND BONE
- The discovery of independent skeletal effects of pituitary hormones highlights the power of integrative bone physiology as it relates to the remodeling of bone and maintenance of its integrity. Bone interacts with not only closely situated organs—bone marrow, fat and muscle—but also with remote organs, such as the pancreas, brain, and kidney. The skeletal role of FSH explains, at least in part, the natural course of bone changes during the perimenopausal transition, together with its expanding function in the pathogenesis of obesity [14] and spikes of cognitive decline during this phase in a woman’s life [13]. Thus, we propose that FSH is an important aging hormone. Furthermore, the opposing skeletal actions of pituitary hormone and the induced master hormones suggest mutually compensatory effects to regulate skeletal homeostasis precisely. For example, hyperthyroid bone loss (low TSH and high T4/T3) could be counteracted by T4/T3-induced TSH-βv secretion from immune cells. In addition, effects of OXT and prolactin on the skeleton mark a potential role for the hormones during pregnancy and lactation. Likewise, the skeletal role of vasopressin suggests that sodium balance in the skeleton, which has not been studied well, also plays an important role in bone remodeling. In all, the exciting new out-of-the-box discoveries not only offer better understanding of bone biology, but also unmask potential therapeutic targets for osteoporosis.
CONCLUSIONS
-
CONFLICTS OF INTEREST
Mone Zaidi is an inventor on issued patents on inhibiting FSH for the prevention and treatment of osteoporosis and obesity (U.S. Patent 8,435,948 and 11,034,761). Mone Zaidi is also an inventor on pending patent application on composition and use of humanized monoclonal anti-FSH antibodies and is co-inventor of a pending patent on the use of FSH as a target for preventing Alzheimer’s disease. These patents are owned by Icahn School of Medicine at Mount Sinai, and Mone Zaidi would be recipient of royalties, per institutional policy. Mone Zaidi also consults for several financial platforms, including Gerson Lehman Group and Guidepoint, on drugs for osteoporosis and genetic bone diseases.
Article information
-
Acknowledgements
- Work at Icahn School of Medicine at Mount Sinai performed at the Center for Translational Medicine and Pharmacology was supported by R01 AG071870 to Mone Zaidi, Tony Yuen, and Se-Min Kim; R01 AG074092 and U01 AG073148 to Tony Yuen and Mone Zaidi; U19 AG060917 to Mone Zaidi and Clifford James Rosen; and R01 DK113627 to Mone Zaidi and Jameel Iqbal. Mone Zaidi also thanks the Harrington Discovery Institute for the Innovator-Scholar Award. Clifford James Rosen acknowledges support from the NIH (P20 GM121301 to Clifford James Rosen).

- 1. Davies T, Marians R, Latif R. The TSH receptor reveals itself. J Clin Invest 2002;110:161–4.ArticlePubMedPMC
- 2. Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, et al. TSH is a negative regulator of skeletal remodeling. Cell 2003;115:151–62.ArticlePubMed
- 3. Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptornull mice. Proc Natl Acad Sci U S A 2002;99:15776–81.ArticlePubMedPMC
- 4. Blair HC, Robinson LJ, Sun L, Isales C, Davies TF, Zaidi M. Skeletal receptors for steroid-family regulating glycoprotein hormones: a multilevel, integrated physiological control system. Ann N Y Acad Sci 2011;1240:26–31.PubMed
- 5. Inoue M, Tawata M, Yokomori N, Endo T, Onaya T. Expression of thyrotropin receptor on clonal osteoblast-like rat osteosarcoma cells. Thyroid 1998;8:1059–64.ArticlePubMed
- 6. Sampath TK, Simic P, Sendak R, Draca N, Bowe AE, O’Brien S, et al. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J Bone Miner Res 2007;22:849–59.ArticlePubMed
- 7. Tsai JA, Janson A, Bucht E, Kindmark H, Marcus C, Stark A, et al. Weak evidence of thyrotropin receptors in primary cultures of human osteoblast-like cells. Calcif Tissue Int 2004;74:486–91.ArticlePubMedPDF
- 8. Bassett JH, Williams AJ, Murphy E, Boyde A, Howell PG, Swinhoe R, et al. A lack of thyroid hormones rather than excess thyrotropin causes abnormal skeletal development in hypothyroidism. Mol Endocrinol 2008;22:501–12.ArticlePubMedPMC
- 9. Sun L, Vukicevic S, Baliram R, Yang G, Sendak R, McPherson J, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci U S A 2008;105:4289–94.ArticlePubMedPMC
- 10. Jayaraman A, Kumar TR. Extra-pituitary expressed follicle-stimulating hormone: is it physiologically important? Biol Reprod 2017;97:622–6.ArticlePubMed
- 11. Kumar TR. Extragonadal FSH receptor: is it real? Biol Reprod 2014;91:99.PubMedPMC
- 12. Zhu D, Li X, Macrae VE, Simoncini T, Fu X. Extragonadal effects of follicle-stimulating hormone on osteoporosis and cardiovascular disease in women during menopausal transition. Trends Endocrinol Metab 2018;29:571–80.ArticlePubMed
- 13. Xiong J, Kang SS, Wang Z, Liu X, Kuo TC, Korkmaz F, et al. FSH blockade improves cognition in mice with Alzheimer’s disease. Nature 2022;603:470–6.ArticlePubMedPMCPDF
- 14. Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 2017;546:107–12.ArticlePubMedPMCPDF
- 15. Ji Y, Liu P, Yuen T, Haider S, He J, Romero R, et al. Epitope-specific monoclonal antibodies to FSHβ increase bone mass. Proc Natl Acad Sci U S A 2018;115:2192–7.ArticlePubMedPMC
- 16. Stilley JA, Christensen DE, Dahlem KB, Guan R, Santillan DA, England SK, et al. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol Reprod 2014;91:74.PubMedPMC
- 17. Yee JB, Hutson JC. Biochemical consequences of follicle-stimulating hormone binding to testicular macrophages in culture. Biol Reprod 1985;32:872–9.ArticlePubMed
- 18. Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med 2010;363:1621–30.ArticlePubMed
- 19. Zhang M, Wang Y, Huan Z, Liu Y, Zhang W, Kong D, et al. FSH modulated cartilage ECM metabolism by targeting the PKA/CREB/SOX9 pathway. J Bone Miner Metab 2021;39:769–79.ArticlePubMedPDF
- 20. Robinson LJ, Tourkova I, Wang Y, Sharrow AC, Landau MS, Yaroslavskiy BB, et al. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem Biophys Res Commun 2010;394:12–7.ArticlePubMedPMC
- 21. Cannon JG, Kraj B, Sloan G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine 2011;53:141–4.ArticlePubMedPMC
- 22. Ulloa-Aguirre A, Zarinan T, Jardon-Valadez E, Gutierrez-Sagal R, Dias JA. Structure-function relationships of the follicle-stimulating hormone receptor. Front Endocrinol (Lausanne) 2018;9:707.ArticlePubMedPMC
- 23. Chida D, Nakagawa S, Nagai S, Sagara H, Katsumata H, Imaki T, et al. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci U S A 2007;104:18205–10.ArticlePubMedPMC
- 24. Zhong Q, Sridhar S, Ruan L, Ding KH, Xie D, Insogna K, et al. Multiple melanocortin receptors are expressed in bone cells. Bone 2005;36:820–31.ArticlePubMed
- 25. Blalock JE. Proopiomelanocortin and the immune-neuroendocrine connection. Ann N Y Acad Sci 1999;885:161–72.ArticlePubMed
- 26. Coss D, Yang L, Kuo CB, Xu X, Luben RA, Walker AM. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. Am J Physiol Endocrinol Metab 2000;279:E1216–25.ArticlePubMed
- 27. Seriwatanachai D, Thongchote K, Charoenphandhu N, Pandaranandaka J, Tudpor K, Teerapornpuntakit J, et al. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone 2008;42:535–46.ArticlePubMed
- 28. Clement-Lacroix P, Ormandy C, Lepescheux L, Ammann P, Damotte D, Goffin V, et al. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology 1999;140:96–105.ArticlePubMed
- 29. Sun L, Tamma R, Yuen T, Colaianni G, Ji Y, Cuscito C, et al. Functions of vasopressin and oxytocin in bone mass regulation. Proc Natl Acad Sci U S A 2016;113:164–9.ArticlePubMedPMC
- 30. Li CH, Simpson ME, Evans HM. Isolation of pituitary follicle-stimulating hormone (FSH). Science 1949;109:445–6.ArticlePubMed
- 31. Gemzell CA, Diczfalusy E, Tillinger G. Clinical effect of human pituitary follicle-stimulating hormone (FSH). J Clin Endocrinol Metab 1958;18:1333–48.ArticlePubMed
- 32. Purves HD, Griesbach WE. The site of thyrotrophin and gonadotrophin production in the rat pituitary studied by McManus-Hotchkiss staining for glycoprotein. Endocrinology 1951;49:244–64.ArticlePubMed
- 33. Purves HD, Griesbach WE. The site of follicle stimulating and luteinizing hormone production in the rat pituitary. Endocrinology 1954;65:785–93.PubMed
- 34. Hisaw CG, Heller EJ, Sevringhaus EL. Does estrogen substitution materially inhibit pituitary gonadotropic potency? Endocrinology 1942;30:309–16.Article
- 35. Byrnes WW, Meyer RK, Finerty JC. Inhibition of gonadotrophic hormone in female parabiotic rats by estrogen and progesterone. Am J Physiol 1951;164:26–30.ArticlePubMed
- 36. Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, et al. FSH directly regulates bone mass. Cell 2006;125:247–60.ArticlePubMed
- 37. Gao J, Tiwari-Pandey R, Samadfam R, Yang Y, Miao D, Karaplis AC, et al. Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone. Endocrinology 2007;148:2613–21.ArticlePubMed
- 38. Morgan I, Coulombe JC, Larsen M, Liu Z, Ferguson VL, Kumar TR. VISIONS: FSH and bone microarchitecture in mice. Mol Reprod Dev 2022;89:315.ArticlePMCPDF
- 39. Liu S, Cheng Y, Xu W, Bian Z. Protective effects of follicle-stimulating hormone inhibitor on alveolar bone loss resulting from experimental periapical lesions in ovariectomized rats. J Endod 2010;36:658–63.ArticlePubMed
- 40. Zhu LL, Tourkova I, Yuen T, Robinson LJ, Bian Z, Zaidi M, et al. Blocking FSH action attenuates osteoclastogenesis. Biochem Biophys Res Commun 2012;422:54–8.ArticlePubMedPMC
- 41. Gera S, Sant D, Haider S, Korkmaz F, Kuo TC, Mathew M, et al. First-in-class humanized FSH blocking antibody targets bone and fat. Proc Natl Acad Sci U S A 2020;117:28971–9.ArticlePubMedPMC
- 42. Geng W, Yan X, Du H, Cui J, Li L, Chen F. Immunization with FSHβ fusion protein antigen prevents bone loss in a rat ovariectomy-induced osteoporosis model. Biochem Biophys Res Commun 2013;434:280–6.ArticlePubMed
- 43. Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, et al. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci U S A 2012;109:14574–9.ArticlePubMedPMC
- 44. Wang J, Zhang W, Yu C, Zhang X, Zhang H, Guan Q, et al. Follicle-stimulating hormone increases the risk of postmenopausal osteoporosis by stimulating osteoclast differentiation. PLoS One 2015;10:e0134986.ArticlePubMedPMC
- 45. Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci U S A 2006;103:14925–30.ArticlePubMedPMC
- 46. Cannon JG, Cortez-Cooper M, Meaders E, Stallings J, Haddow S, Kraj B, et al. Follicle-stimulating hormone, interleukin-1, and bone density in adult women. Am J Physiol Regul Integr Comp Physiol 2010;298:R790–8.ArticlePubMedPMC
- 47. Wu Y, Torchia J, Yao W, Lane NE, Lanier LL, Nakamura MC, et al. Bone microenvironment specific roles of ITAM adapter signaling during bone remodeling induced by acute estrogen-deficiency. PLoS One 2007;2:e586.ArticlePubMedPMC
- 48. Garton M, Martin J, New S, Lee S, Loveridge N, Milne J, et al. Bone mass and metabolism in women aged 45-55. Clin Endocrinol (Oxf) 1996;44:563–70.ArticlePubMedPDF
- 49. Steinberg KK, Freni-Titulaer LW, DePuey EG, Miller DT, Sgoutas DS, Coralli CH, et al. Sex steroids and bone density in premenopausal and perimenopausal women. J Clin Endocrinol Metab 1989;69:533–9.ArticlePubMed
- 50. Johnston CC Jr, Hui SL, Witt RM, Appledorn R, Baker RS, Longcope C. Early menopausal changes in bone mass and sex steroids. J Clin Endocrinol Metab 1985;61:905–11.ArticlePubMed
- 51. Ebeling PR, Atley LM, Guthrie JR, Burger HG, Dennerstein L, Hopper JL, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab 1996;81:3366–71.ArticlePubMed
- 52. Guthrie JR, Ebeling PR, Hopper JL, Dennerstein L, Wark JD, Burger HG. Bone mineral density and hormone levels in menopausal Australian women. Gynecol Endocrinol 1996;10:199–205.ArticlePubMed
- 53. Peichl P, Griesmacher A, Pointinger P, Marteau R, Hartl W, Gruber W, et al. Association between female sex hormones and biochemical markers of bone turnover in peri- and postmenopausal women. Calcif Tissue Int 1998;62:388–94.ArticlePubMedPDF
- 54. Devleta B, Adem B, Senada S. Hypergonadotropic amenorrhea and bone density: new approach to an old problem. J Bone Miner Metab 2004;22:360–4.ArticlePubMedPDF
- 55. Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int 2003;14:191–7.ArticlePubMedPDF
- 56. Veldhuis-Vlug AG, Woods GN, Sigurdsson S, Ewing SK, Le PT, Hue TF, et al. Serum FSH Is associated with BMD, bone marrow adiposity, and body composition in the AGES-Reykjavik study of older adults. J Clin Endocrinol Metab 2021;106:e1156–69.ArticlePubMedPMCPDF
- 57. Xu ZR, Wang AH, Wu XP, Zhang H, Sheng ZF, Wu XY, et al. Relationship of age-related concentrations of serum FSH and LH with bone mineral density, prevalence of osteoporosis in native Chinese women. Clin Chim Acta 2009;400:8–13.ArticlePubMed
- 58. Gallagher CM, Moonga BS, Kovach JS. Cadmium, follicle-stimulating hormone, and effects on bone in women age 42-60 years, NHANES III. Environ Res 2010;110:105–11.ArticlePubMed
- 59. Adami S, Bianchi G, Brandi ML, Giannini S, Ortolani S, DiMunno O, et al. Determinants of bone turnover markers in healthy premenopausal women. Calcif Tissue Int 2008;82:341–7.ArticlePubMedPDF
- 60. Rendina D, Gianfrancesco F, De Filippo G, Merlotti D, Esposito T, Mingione A, et al. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. Eur J Endocrinol 2010;163:165–72.ArticlePubMed
- 61. van der Deure WM, Uitterlinden AG, Hofman A, Rivadeneira F, Pols HA, Peeters RP, et al. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: the Rotterdam study. Clin Endocrinol (Oxf) 2008;68:175–81.ArticlePubMed
- 62. Morris MS. The association between serum thyroid-stimulating hormone in its reference range and bone status in postmenopausal American women. Bone 2007;40:1128–34.ArticlePubMed
- 63. Yang L, Wang H, Guo J, Zheng G, Wei D, Zhang T. Low normal TSH levels and thyroid autoimmunity are associated with an increased risk of osteoporosis in euthyroid postmenopausal women. Endocr Metab Immune Disord Drug Targets 2021;21:859–65.ArticlePubMedPDF
- 64. Noh HM, Park YS, Lee J, Lee W. A cross-sectional study to examine the correlation between serum TSH levels and the osteoporosis of the lumbar spine in healthy women with normal thyroid function. Osteoporos Int 2015;26:997–1003.ArticlePubMedPDF
- 65. Kim DJ, Khang YH, Koh JM, Shong YK, Kim GS. Low normal TSH levels are associated with low bone mineral density in healthy postmenopausal women. Clin Endocrinol (Oxf) 2006;64:86–90.ArticlePubMed
- 66. Murphy E, Gluer CC, Reid DM, Felsenberg D, Roux C, Eastell R, et al. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab 2010;95:3173–81.ArticlePubMed
- 67. Grimnes G, Emaus N, Joakimsen RM, Figenschau Y, Jorde R. The relationship between serum TSH and bone mineral density in men and postmenopausal women: the Tromso study. Thyroid 2008;18:1147–55.ArticlePubMed
- 68. Aubert CE, Floriani C, Bauer DC, da Costa BR, Segna D, Blum MR, et al. Thyroid function tests in the reference range and fracture: individual participant analysis of prospective cohorts. J Clin Endocrinol Metab 2017;102:2719–28.ArticlePubMedPMCPDF
- 69. Bauer DC, Ettinger B, Nevitt MC, Stone KL; Study of Osteoporotic Fractures Research Group. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med 2001;134:561–8.ArticlePubMed
- 70. Abrahamsen B, Jorgensen HL, Laulund AS, Nybo M, Brix TH, Hegedus L. Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures-the OPENTHYRO register cohort. J Bone Miner Res 2014;29:2040–50.ArticlePubMed
- 71. Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR, et al. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA 2015;313:2055–65.ArticlePubMedPMC
- 72. Segna D, Bauer DC, Feller M, Schneider C, Fink HA, Aubert CE, et al. Association between subclinical thyroid dysfunction and change in bone mineral density in prospective cohorts. J Intern Med 2018;283:56–72.ArticlePubMedPMCPDF
- 73. Kim EH, Jeon YK, Pak K, Kim IJ, Kim SJ, Shin S, et al. Effects of thyrotropin suppression on bone health in menopausal women with total thyroidectomy. J Bone Metab 2019;26:31–8.ArticlePubMedPMCPDF
- 74. Hawkins Carranza F, Guadalix Iglesias S, Luisa De Mingo Dominguez M, Martin-Arriscado Arroba C, Lopez Alvarez B, Allo Miguel G, et al. Trabecular bone deterioration in differentiated thyroid cancer: impact of long-term TSH suppressive therapy. Cancer Med 2020;9:5746–55.ArticlePubMedPMCPDF
- 75. Lee SJ, Kim KM, Lee EY, Song MK, Kang DR, Kim HC, et al. Low normal TSH levels are associated with impaired BMD and hip geometry in the elderly. Aging Dis 2016;7:734–43.ArticlePubMedPMC
- 76. Kim SM, Ryu V, Miyashita S, Korkmaz F, Lizneva D, Gera S, et al. Thyrotropin, hyperthyroidism, and bone mass. J Clin Endocrinol Metab 2021;106:e4809–21.ArticlePubMedPMCPDF
- 77. Martini G, Gennari L, De Paola V, Pilli T, Salvadori S, Merlotti D, et al. The effects of recombinant TSH on bone turnover markers and serum osteoprotegerin and RANKL levels. Thyroid 2008;18:455–60.ArticlePubMed
- 78. Mazziotti G, Sorvillo F, Piscopo M, Cioffi M, Pilla P, Biondi B, et al. Recombinant human TSH modulates in vivo C-telopeptides of type-1 collagen and bone alkaline phosphatase, but not osteoprotegerin production in postmenopausal women monitored for differentiated thyroid carcinoma. J Bone Miner Res 2005;20:480–6.ArticlePubMed
- 79. Karga H, Papaioannou G, Polymeris A, Papamichael K, Karpouza A, Samouilidou E, et al. The effects of recombinant human TSH on bone turnover in patients after thyroidectomy. J Bone Miner Metab 2010;28:35–41.ArticlePubMedPDF
- 80. Sun L, Zhu LL, Lu P, Yuen T, Li J, Ma R, et al. Genetic confirmation for a central role for TNFα in the direct action of thyroid stimulating hormone on the skeleton. Proc Natl Acad Sci U S A 2013;110:9891–6.ArticlePubMedPMC
- 81. Baliram R, Sun L, Cao J, Li J, Latif R, Huber AK, et al. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. J Clin Invest 2012;122:3737–41.ArticlePubMedPMC
- 82. Williams GR, Bassett JH. Thyroid diseases and bone health. J Endocrinol Invest 2018;41:99–109.ArticlePubMedPMCPDF
- 83. Hase H, Ando T, Eldeiry L, Brebene A, Peng Y, Liu L, et al. TNFalpha mediates the skeletal effects of thyroid-stimulating hormone. Proc Natl Acad Sci U S A 2006;103:12849–54.PubMedPMC
- 84. Diez JJ, Hernanz A, Medina S, Bayon C, Iglesias P. Serum concentrations of tumour necrosis factor-alpha (TNF-alpha) and soluble TNF-alpha receptor p55 in patients with hypothyroidism and hyperthyroidism before and after normalization of thyroid function. Clin Endocrinol (Oxf) 2002;57:515–21.PubMed
- 85. Yamoah K, Brebene A, Baliram R, Inagaki K, Dolios G, Arabi A, et al. High-mobility group box proteins modulate tumor necrosis factor-alpha expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Mol Endocrinol 2008;22:1141–53.PubMedPMC
- 86. Klein JR. Biological impact of the TSHβ splice variant in health and disease. Front Immunol 2014;5:155.ArticlePubMedPMC
- 87. Baliram R, Chow A, Huber AK, Collier L, Ali MR, Morshed SA, et al. Thyroid and bone: macrophage-derived TSH-β splice variant increases murine osteoblastogenesis. Endocrinology 2013;154:4919–26.ArticlePubMedPMCPDF
- 88. Wang HC, Dragoo J, Zhou Q, Klein JR. An intrinsic thyrotropin-mediated pathway of TNF-alpha production by bone marrow cells. Blood 2003;101:119–23.PubMed
- 89. Liu C, Miao J, Liu X, Zhao Z, Kou T, Liu J, et al. HPT axis-independent TSHβ splice variant regulates the synthesis of thyroid hormone in mice. Mol Med Rep 2019;19:4514–22.ArticlePubMed
- 90. Baliram R, Latif R, Morshed SA, Zaidi M, Davies TF. T3 regulates a human macrophage-derived TSH-β splice variant: implications for human bone biology. Endocrinology 2016;157:3658–67.ArticlePubMedPMCPDF
- 91. van der Poll T, Romijn JA, Wiersinga WM, Sauerwein HP. Tumor necrosis factor: a putative mediator of the sick euthyroid syndrome in man. J Clin Endocrinol Metab 1990;71:1567–72.ArticlePubMed
- 92. Montufar-Solis D, Klein JR. Splenic leukocytes traffic to the thyroid and produce a novel TSHβ isoform during acute listeria monocytogenes infection in mice. PLoS One 2016;11:e0146111.ArticlePubMedPMC
- 93. Ramajayam G, Vignesh RC, Karthikeyan S, Kumar KS, Karthikeyan GD, Veni S, et al. Regulation of insulin-like growth factors and their binding proteins by thyroid stimulating hormone in human osteoblast-like (SaOS2) cells. Mol Cell Biochem 2012;368:77–88.ArticlePubMedPDF
- 94. Baliram R, Latif R, Berkowitz J, Frid S, Colaianni G, Sun L, et al. Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures. Proc Natl Acad Sci U S A 2011;108:16277–82.ArticlePubMedPMC
- 95. Boutin A, Gershengorn MC, Neumann S. β-Arrestin 1 in thyrotropin receptor signaling in bone: studies in osteoblast-like cells. Front Endocrinol (Lausanne) 2020;11:312.ArticlePubMedPMC
- 96. Cassier E, Gallay N, Bourquard T, Claeysen S, Bockaert J, Crepieux P, et al. Phosphorylation of β-arrestin2 at Thr383 by MEK underlies β-arrestin-dependent activation of Erk1/2 by GPCRs. Elife 2017;6:e23777.ArticlePubMedPMCPDF
- 97. Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci U S A 2010;107:8782–7.ArticlePubMedPMC
- 98. Tourkova IL, Liu L, Sutjarit N, Larrouture QC, Luo J, Robinson LJ, et al. Adrenocorticotropic hormone and 1,25-dihydroxyvitamin D3 enhance human osteogenesis in vitro by synergistically accelerating the expression of bone-specific genes. Lab Invest 2017;97:1072–83.ArticlePubMedPMCPDF
- 99. Sadeghi F, Vahednia E, Naderi Meshkin H, Kerachian MA. The effect of adrenocorticotropic hormone on alpha-2-macroglobulin in osteoblasts derived from human mesenchymal stem cells. J Cell Mol Med 2020;24:4784–90.ArticlePubMedPMCPDF
- 100. Kerachian MA, Cournoyer D, Harvey EJ, Chow TY, Begin LR, Nahal A, et al. New insights into the pathogenesis of glucocorticoid-induced avascular necrosis: microarray analysis of gene expression in a rat model. Arthritis Res Ther 2010;12:R124.ArticlePubMedPMC
- 101. Minetto M, Reimondo G, Osella G, Ventura M, Angeli A, Terzolo M. Bone loss is more severe in primary adrenal than in pituitary-dependent Cushing’s syndrome. Osteoporos Int 2004;15:855–61.ArticlePubMedPDF
- 102. Sato T, Iwata T, Usui M, Kokabu S, Sugamori Y, Takaku Y, et al. Bone phenotype in melanocortin 2 receptor-deficient mice. Bone Rep 2020;13:100713.ArticlePubMedPMC
- 103. Bouillon R. Growth hormone and bone. Horm Res 1991;36 Suppl 1:49–55.ArticlePubMed
- 104. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 2001;229:141–62.ArticlePubMed
- 105. Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A 1999;96:7324–9.ArticlePubMedPMC
- 106. Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A 1999;96:7088–92.ArticlePubMedPMC
- 107. Yakar S, Rosen CJ, Bouxsein ML, Sun H, Mejia W, Kawashima Y, et al. Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB J 2009;23:709–19.ArticlePubMedPMCPDF
- 108. Ohlsson C, Mohan S, Sjogren K, Tivesten A, Isgaard J, Isaksson O, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev 2009;30:494–535.ArticlePubMedPMC
- 109. Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology 2007;148:5706–15.PubMed
- 110. Wysolmerski JJ. The evolutionary origins of maternal calcium and bone metabolism during lactation. J Mammary Gland Biol Neoplasia 2002;7:267–76.PubMed
- 111. Sowers M, Eyre D, Hollis BW, Randolph JF, Shapiro B, Jannausch ML, et al. Biochemical markers of bone turnover in lactating and nonlactating postpartum women. J Clin Endocrinol Metab 1995;80:2210–6.ArticlePubMed
- 112. Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology 2003;144:2291–6.ArticlePubMed
- 113. Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A 1996;93:11699–704.ArticlePubMedPMC
- 114. Copland JA, Ives KL, Simmons DJ, Soloff MS. Functional oxytocin receptors discovered in human osteoblasts. Endocrinology 1999;140:4371–4.ArticlePubMed
- 115. Colucci S, Colaianni G, Mori G, Grano M, Zallone A. Human osteoclasts express oxytocin receptor. Biochem Biophys Res Commun 2002;297:442–5.ArticlePubMed
- 116. Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, et al. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci U S A 2009;106:7149–54.ArticlePubMedPMC
- 117. Elabd SK, Sabry I, Hassan WB, Nour H, Zaky K. Possible neuroendocrine role for oxytocin in bone remodeling. Endocr Regul 2007;41:131–41.PubMed
- 118. Sun L, Lizneva D, Ji Y, Colaianni G, Hadelia E, Gumerova A, et al. Oxytocin regulates body composition. Proc Natl Acad Sci U S A 2019;116:26808–15.ArticlePubMedPMC
- 119. Liu X, Shimono K, Zhu LL, Li J, Peng Y, Imam A, et al. Oxytocin deficiency impairs maternal skeletal remodeling. Biochem Biophys Res Commun 2009;388:161–6.ArticlePubMed
- 120. Tamma R, Sun L, Cuscito C, Lu P, Corcelli M, Li J, et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc Natl Acad Sci U S A 2013;110:18644–9.ArticlePubMedPMC
References
Figure & Data
References
Citations

- New tools for bone health assessment in secreting pituitary adenomas
Meliha Melin Uygur, Stefano Frara, Luigi di Filippo, Andrea Giustina
Trends in Endocrinology & Metabolism.2023; 34(4): 231. CrossRef - A Causality between Thyroid Function and Bone Mineral Density in Childhood: Abnormal Thyrotropin May Be Another Pediatric Predictor of Bone Fragility
Dongjin Lee, Moon Ahn
Metabolites.2023; 13(3): 372. CrossRef - The mechanism of oxytocin and its receptors in regulating cells in bone metabolism
Liu Feixiang, Feng Yanchen, Li Xiang, Zhang Yunke, Miao Jinxin, Wang Jianru, Lin Zixuan
Frontiers in Pharmacology.2023;[Epub] CrossRef - To investigate the mechanism of Yiwei Decoction in the treatment of premature ovarian insufficiency-related osteoporosis using transcriptomics, network pharmacology and molecular docking techniques
Weisen Fan, Yan Meng, Jing Zhang, Muzhen Li, Yingjie Zhang, Xintian Qu, Xin Xiu
Scientific Reports.2023;[Epub] CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite