Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(3); 2022 > Article
-
Original ArticleThyroid Seaweed and Iodine Intakes and SLC5A5 rs77277498 in Relation to Thyroid Cancer
 Keypoint
Keypoint
This study aimed to elucidate the associations among dietary seaweed (gim and miyeok/dashima) and iodine intake, the rs77277498 polymorphism of the SLC5A5 gene encoding the sodium/iodine symporter, and thyroid cancer risk in a Korean population. The authors found an effect modification of rs77277498 on the association between dietary gim and iodine intake and thyroid cancer risk. This study provided evidence to support the putative effects of dietary gim and iodine intake against thyroid cancer risk, and the strength of this association can be modified by the rs77277498 genotype. -
Tung Hoang1*
 , Eun Kyung Lee2*
, Eun Kyung Lee2* , Jeonghee Lee1, Yul Hwangbo2, Jeongseon Kim1
, Jeonghee Lee1, Yul Hwangbo2, Jeongseon Kim1
-
Endocrinology and Metabolism 2022;37(3):513-523.
DOI: https://doi.org/10.3803/EnM.2021.1306
Published online: May 24, 2022
1Department of Cancer Biomedical Science, National Cancer Center Graduate School of Cancer Science and Policy, Goyang, Korea
2Center for Thyroid Cancer, National Cancer Center, Goyang, Korea
- Corresponding author: Jeongseon Kim. Department of Cancer Biomedical Science, National Cancer Center Graduate School of Cancer Science and Policy, 323 Ilsan-ro, Ilsandong-gu, Goyang 10408, Korea Tel: +82-31-920-2570, Fax: +82-31-920-2579, E-mail: jskim@ncc.re.kr
- *These authors contributed equally to this work.
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- This study aims to elucidate the associations among dietary seaweed (gim and miyeok/dashima) and iodine intakes, the rs77277498 polymorphism of the SLC5A5 gene codifying the sodium/iodine symporter, and thyroid cancer risk in a Korean population.
-
Methods
- We conducted a case-control study of 117 thyroid cancer cases and 173 controls who participated in the Cancer Screenee Cohort between 2002 and 2014 at the National Cancer Center, Korea. The amount of seaweed and iodine consumption (g/day) was estimated using the residual energy adjustment method. We calculated odds ratios (ORs) and their 95% confidence intervals (CIs) using a multivariable logistic regression model for the separate and combined effect of dietary iodine-based intake and SLC5A5 polymorphism (rs77277498, C>G) on thyroid cancer.
-
Results
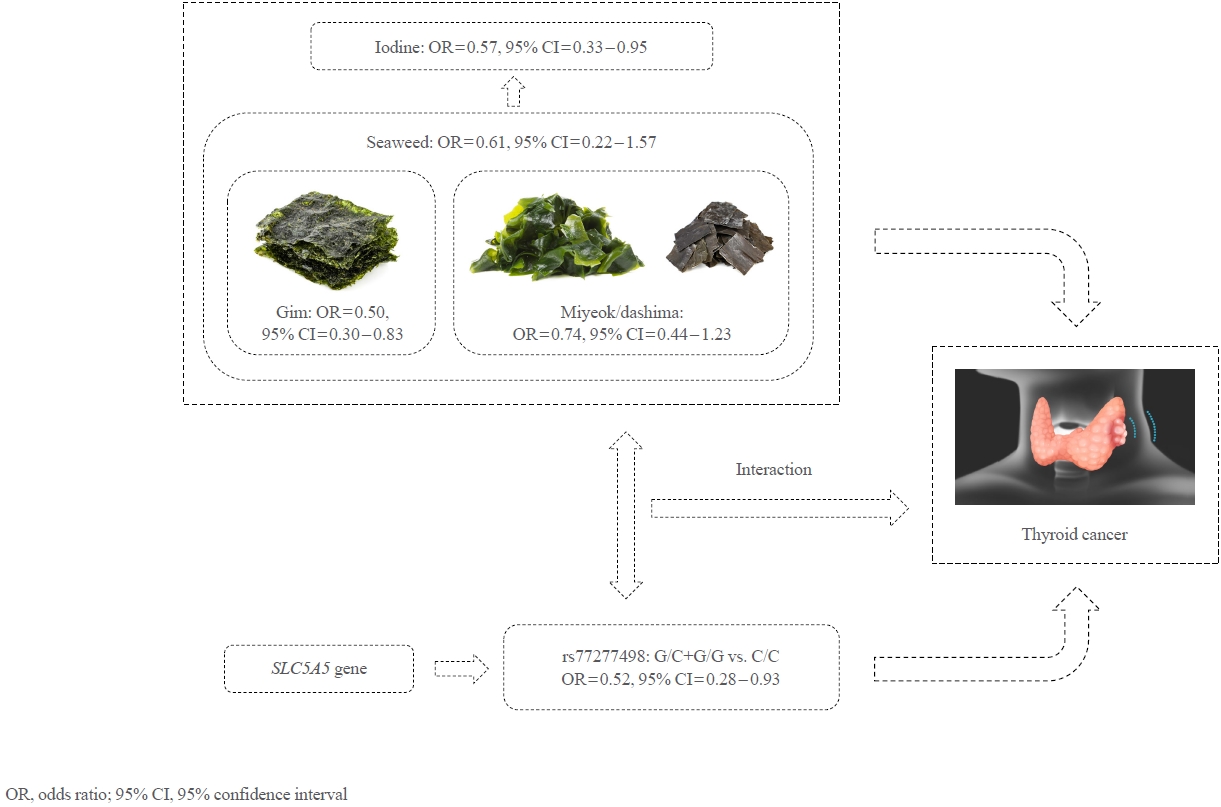
- Dietary gim and iodine intakes were inversely associated with thyroid cancer, with ORs of 0.50 (95% CI, 0.30 to 0.83) and 0.57 (95% CI, 0.35 to 0.95), respectively, whereas the associations for dietary miyeok/dashima and total seaweed intakes were not significant. However, compared with individuals carrying the C/C genotype of the rs77277498 polymorphism with a low intake of all dietary factors, those carrying the G allele with a high intake had a lower risk of thyroid cancer, with ORs of 0.25 (95% CI, 0.10 to 0.56), 0.31 (95% CI, 0.12 to 0.77), 0.26 (95% CI, 0.10 to 0.62), and 0.30 (95% CI, 0.12 to 0.73) for the consumption of gim, miyeok/dashima, total seaweed, and iodine, respectively.
-
Conclusion
- In summary, our results supported the evidence of the protective effects of dietary gim and iodine intake against thyroid cancer risk, and this association can be strengthened by SLC5A5 rs77277498 genotypes.
- Worldwide, thyroid cancer is the ninth most common cancer overall and the most common malignancy of the endocrine system over the past decades [1]. In 2020, approximately 586,000 new cases were estimated, accounting for 3.0% of the total cancer burden [1]. In Korea, thyroid cancer ranks sixth in males but second in females, with age-standardized incidence rates of 22.1 per 100,000 and 66.3 per 100,000, respectively [2]. In addition to the increased detection of thyroid cancer, exposure to ionizing radiation during childhood and adolescence is the only well-established risk factor for different types of thyroid carcinoma [3,4]. In terms of other factors that contribute to thyroid cancer, iodine is a vital micronutrient that is required for the synthesis of thyroid-stimulating hormones [5]. Insufficient intake of iodine therefore adversely affects thyroid function and may result in an increased risk of thyroid cancer development.
- Approximately 65.6% of the iodine intake among Koreans is estimated to come from seaweeds [6,7]. In the traditional Korean diet, seaweed is commonly provided as dried or water-containing forms, including gim (Porphyra sp. laver), miyeok (Undaria pinnatifida sea mustard), and dashima (Laminaria sp. sea tangle) [8,9]. In addition to iodine, other bioactive molecules in seaweed, such as polysaccharides, proteins, polyphenols, carotenoids, and n-3 polyunsaturated fatty acids from seaweed are a beneficial source for human health [10]. Furthermore, in vitro and in vivo models have been suggested the anticarcinogenic effects of these components, which include regulating tumor metabolism, apoptosis, and cell proliferation [9,11,12]. However, epidemiological studies regarding the effect of dietary seaweed and iodine on thyroid cancer in the Korean population have been limited.
- Furthermore, the sodium/iodine symporter (encoded by the SLC5A5 gene) is an intrinsic membrane protein that is involved in the metabolism of iodine from the bloodstream into the follicular cells of the thyroid using the sodium gradient generated by Na+/K+-ATPase [13-15]. SLC5A5 expression is lower in thyroid carcinomas or adenomas than in normal adjacent thyroid tissue [14]. Additionally, SLC5A5 expression is suggested to differentiate follicular adenomas from follicular carcinomas in cumulative studies [13,16]. Given the important role of SLC5A5 in the iodine uptake pathway and its effect on thyroid cancer prognosis, this study aimed to examine the associations of dietary seaweed and iodine intakes in relation to the risk of thyroid cancer in Korean adults to determine whether this association was modified by SLC5A5 polymorphisms.
INTRODUCTION
- Study population
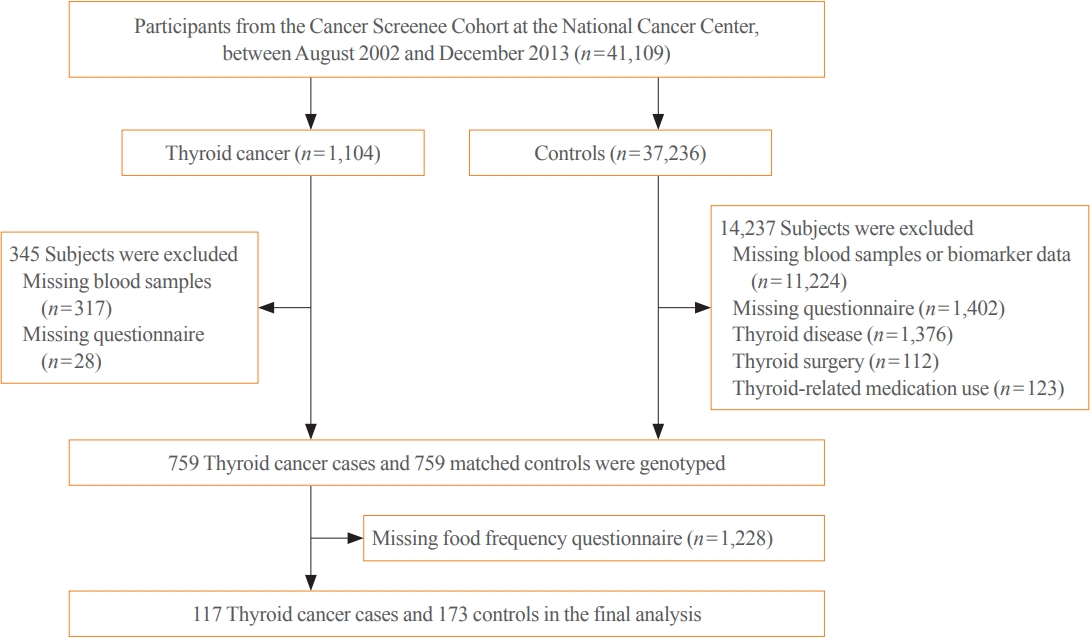
- This hospital-based case-control study recruited a total of 41,109 participants who underwent health screening examinations, including thyroid ultrasonography, at the National Cancer Center (NCC), South Korea, between 2002 and 2014 (Fig. 1). Details of this project have been described elsewhere [17]. At baseline, all subjects provided the written consent form and completed a general questionnaire and a semiquantitative food frequency questionnaire (SQFFQ) about their demographics, lifestyles, and dietary consumption. The cases were defined as having a diagnosis of thyroid cancer, with the International Classification of Diseases, Tenth Revision (ICD-10) code of C73 according to data linkage to the Korea Central Cancer Registry. Among the 1,104 subjects diagnosed with thyroid cancer, we excluded 345 subjects who had missing blood samples (n=317) or did not complete the general questionnaire (n=28). Additionally, among 37,236 potential controls, we excluded those who did not complete the general questionnaire or with missing biomarker data or a history of thyroid-related disease, surgery, or medication use. A total of 759 cases and 759 ageand sex-matched controls who were free of other cancer types were selected for genotyping. General characteristics of total genotyping population were described elsewhere [18]. Of these, 117 cases and 173 controls with available information on the SQFFQ were included in the final analysis.
- Data collection
- Demographic information including age (years), sex (male/female), family history of thyroid cancer (yes/no), marital status (married/other), educational level (≤middle school, high school, and ≥college), occupation (housewives, professional, and office worker; sales and service; and agriculture, laborer, unemployed, and others), monthly income (<$1,667, $1,667–$3,333, and >$3,333), smoking status (ever/never), alcohol consumption (ever/never), and regular exercise (yes/no) were collected using a generally interviewer-administered questionnaire. Height and weight were measured using an Inbody version 3.0 (Biospace, Seoul, Korea) body composition analyzer or an X-SCAN PLUS II Body Composition Analyzer (Jawon Medical, Gyeongsan, Korea). Body mass index (BMI) was calculated as the ratio of weight (kg) to height squared (m2) and classified into normal (<23 kg/m2), overweight (23–24.9 kg/m2), and obese (≥25 kg/m2) groups.
- Regarding dietary intake, participants were asked about their average consumption frequency for each of 106 food items in the SQFFQ during the previous year from nine frequency categories (never or rarely, once a month, 2–3 times per month, once or twice a week, 3–4 times per week, 5–6 times per week, once a day, twice a day, and 3 times per day) and were then asked about usual portion sizes using three portion size categories (small, medium, and large) [19]. Of the 106 food items, two seaweed items, including gim and miyeok/dashima, could be chosen in the SQFFQ. Seaweed consumption (g/day) of these two items and iodine intake (μg/day) from the whole diet were calculated using the Computer-Aided Nutritional Analysis Program (CAN-Pro) version 4.0 (Korean Nutrition Information Center, Seoul, Korea). In nutritional research, consumption of a diet component will result in a greater total energy intake and quantity of foods and thereby affect several important factors, such as body size and composition [20]. To account for the effect of a single food that is independent of total energy intake, consumption was estimated using the residual energy adjustment method and categorized into low and high intake based on the median value [21]. In particular, we first obtained residuals from the regression model with total energy as the independent variable and absolute intake of gim, miyeok/dashima, and iodine separately. The adjusted intake of these components was then calculated as the predicted values from the model of average energy intake as the independent variable and the residual and coefficient values above as intercepts [21].
- Genotyping and imputation
- The study participants were genotyped using the Infinium OncoArray-500K BeadChip (Illumina Inc., San Diego, CA, USA) with 499,170 single nucleotide polymorphisms (SNPs) (275,691 genome-wide tag SNPs and 223,479 cancer-specific SNPs). After quality control in PLINK version 1.07 for monomorphic variants (minor allele frequency [MAF]=0), MAF <0.01, call rate <95%, and deviation from Hardy-Weinberg equilibrium (P<1×10−6), a total of 345,675 SNPs remained. The data were then imputed using SHAPEIT (v2 r837) [22] and IMPUTE2 (2.3.2) [23], and 5,087,097 SNPs were available for further analysis. In this study, we selected rs77277498 (C>G) of the SLC5A5 gene for the final analysis.
- Statistical analysis
- The comparison between thyroid cancer cases and controls was analyzed using Student’s t test for continuous variables and the chi-square test (or Fisher’s exact test for expected cell frequencies <5) for categorical variables. A logistic regression analysis was implemented to assess the risk of thyroid cancer and calculate odds ratios (ORs) and 95% confidence intervals (95% CIs) for the measurement of associations. The multivariable model was adjusted for age, sex, family history of thyroid cancer, smoking status, alcohol consumption, and BMI. Dietary seaweed (including gim and miyeok/dashima) and iodine intakes on thyroid cancer risk were compared by quartile groups of high (greater than median amount) and low (less than the median amount) consumption. The association of each SNP with thyroid cancer and its interaction effect on dietary consumption and thyroid cancer association were examined in a dominant model. All statistical analyses for the present study were performed using R version 3.6.0 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
- Ethical statement
- All participants provided written informed consent, and the study protocol was approved by the Institutional Review Board of the NCC (IRB No. NCC2016-0088).
METHODS
- Our study included 290 subjects comprising 117 thyroid cancer cases and 173 controls. Individuals with thyroid cancer were more likely to be females (P=0.001) and nonsmokers (P=0.016) (Table 1), whereas age, family history of thyroid cancer, marital status, educational level, occupation, monthly income, smoking status, alcohol consumption, regular exercise, and BMI were equally distributed between the patients and controls (P>0.05). Compared to those without dietary information, the current study population differed in terms of age, sex, educational level, monthly income, smoking status, and alcohol consumption (P<0.05) (Supplemental Table S1). Of these, marital status was not significantly different among cases (P=0.587), whereas family history was additionally different among controls with and without SQFFQ (P=0.022). In addition, although free thyroxine and thyroid stimulating hormone were measured at the health examination date for many participants who were genotyped, those for most of the participants in our final analysis were missing (Supplemental Table S2).
- The characteristics of diet consumption and SLC5A5 variants in thyroid cancer cases and controls are presented in Table 2. The daily amount of gim intake was greater in the healthy subjects than in the cancer subjects (P=0.017), whereas the consumption of miyeok/dashima (P=0.811), total seaweed (P=0.338), and iodine (P=0.231) was comparable between the two groups. Additionally, the genotypes of the rs77277498 (P=0.027) variant were unequally distributed between the two groups.
- Table 3 shows the ORs and 95% CIs of thyroid cancer risk for dietary seaweed (including gim and miyeok/dashima) and iodine intakes and SLC5A5 variants. The higher gim and iodine intake quartiles were inversely associated with thyroid cancer risk compared with the lower quartiles, with adjusted ORs of 0.50 (95% CI, 0.30 to 0.83) and 0.57 (95% CI, 0.33 to 0.95), respectively. In contrast, the intake amount of miyeok/dashima was not significantly associated with thyroid cancer risk (OR, 0.74; 95% CI, 0.44 to 1.23), whereas total seaweed consumption was borderline associated with thyroid cancer (OR, 0.61; 95% CI, 0.36 to 1.02). In addition, gim and miyeok/dashima intake frequency did not differ between cancer and noncancer subjects. Besides, a significant association was observed for rs77277498 (Table 3). Those carrying a G allele (G/C+G/G) had a decreased risk of thyroid cancer compared with those carrying the C/C genotype in the dominant model (OR, 0.52; 95% CI, 0.28 to 0.93).
- Table 4 presents the results for the combined effects of dietary seaweed and iodine and a genetic variant of rs77277498 on thyroid cancer risk. The inverse association between iodine-based dietary intake and thyroid cancer seems to be stronger among G allele carriers than among C/C heterogenous carriers. In the multivariable model, those carrying the G allele with a high consumption of total seaweed, as well as its different forms, including gim and miyeok/dashima, had a significantly lower thyroid cancer risk than those not carrying the minor allele with a low intake, with ORs of 0.26 (95% CI, 0.10 to 0.62), 0.25 (95% CI, 0.10 to 0.56), and 0.31 (95% CI, 0.12 to 0.77), respectively. Regarding intake frequency, those carrying the G allele had a lower risk of thyroid cancer than those who did not (OR, 0.48; 95% CI, 0.23 to 0.99) among subjects who consumed fewer than three times of gim per month. Furthermore, compared with the G allele noncarrier subgroup with a low iodine intake, other subgroups were associated with a lower risk of thyroid cancer, with ORs of 0.42 (95% CI, 0.18 to 0.93) for G/G+G/C carriers with a low intake, 0.51 (95% CI, 0.27 to 0.93) for C/C carriers with a high intake, and 0.30 (95% CI, 0.12 to 0.73) for G/G+G/C carriers with a high intake. The interaction between gim intake frequency and the rs77277498 genotype was significant (P=0.02).
RESULTS
- In this study, we found that high intakes of gim and iodine were associated with a decreased risk of thyroid cancer. In addition, our study results supported a putative role of the rs77277498 polymorphism in the SLC5A5 gene in the reduction in thyroid cancer risk. The G/G+G/C carriers with a high intake of these dietary factors had a lower risk of thyroid cancer than those carrying C/C genotype with a low intake. Additionally, we observed significantly interactive effects between gim intake frequency and rs77277498 in relation to thyroid cancer risk.
- Previous studies have investigated the effect of seaweed consumption on the development of thyroid cancer in Japanese women. Data from the Japan Public Health Center-based Prospective Study revealed a positive association between frequent seaweed intake (almost daily vs. ≤2 days/week) and papillary carcinoma (hazard ratio [HR], 1.71; 95% CI, 1.01 to 2.90) but not total thyroid cancer (HR, 1.41; 95% CI, 0.86 to 2.32). Despite the higher overall incidence rate of thyroid cancer in the Japan Collaborative Cohort Study than in the Japan Public Health Center-based Prospective Study (20.9 vs. 17.5 per 100,000 person-years), seaweed consumption was still not significantly associated with either papillary carcinoma (HR, 1.15; 95% CI, 0.69 to 1.89) or total thyroid cancer (HR, 0.94; 95% CI, 0.51 to 1.74). In the current study, we further assessed total seaweed consumption in terms of both frequency and quantitative measurement of grams per day. However, we did not find any associations between total seaweed intake and the risk of thyroid cancer.
- Regarding the major types of seaweed intake in Koreans, the chemopreventive effect of gim has been proposed to be associated with the anticancer effects of the polysaccharides, phospholipids, sterol, and peptides found in Porphyra sp. on apoptosis and cell proliferation inhibition [24]. This effect was evaluated in different human cell lines of hepatic carcinoma, cervical cancer, breast carcinoma, and gastric cancer [24]. In this study, miyeok/dashima consumption was not associated with a reduced risk of thyroid cancer. This might be due to the low-frequency intake in this population, with approximately 60% of the study participants consuming miyeok/dashima no more than three times per month. However, fucoidan from U. pinnatifida (miyeok) or Laminaria sp. (dashima) has been proven to exert cytotoxic activity against various cancer types including breast, colon, hepatocellular, lung, and prostate cancer cells, by inhibiting proliferation and inducing apoptosis via both mitochondrial-dependent and death receptor rough extrinsic-related pathways [25-30]. In addition, similar anticancer effects have been reported for bioactive compounds extracted from Laminaria japonica, such as glycoproteins, polysaccharides, laminarin, fucoxanthin, and phlorotannin [31-35].
- Furthermore, iodine is an essential component for the synthesis of thyroid hormones [36]. Iodine deficiency has been hypothesized to induce thyroid hyperplasia and increase aneuploidy, thereby eliciting chromosomal changes in the thyroid [36]. Additionally, iodine deficiency together with epidermal growth factor and insulin-like growth factor I is suggested to cause chronic thyroid-stimulating hormone overstimulation, resulting in thyroid tumors [36]. Pooled estimates from a meta-analysis showed that a high intake of iodine was associated with a lower risk of thyroid cancer (OR, 0.74; 95% CI, 0.60 to 0.92), whereas iodine deficiency may not be significantly related to an increased risk (OR, 1.22; 95% CI, 0.94 to 1.58) [37]. Despite the limited number of individual studies in the meta-analysis and the different amounts of iodine intake, the findings appeared to be consistent with our results. In contrast, several studies have indicated the potentially harmful effects of excessive iodine intake regarding hypothyroidism, as excessive intake triggers the Wolff–Chaikoff effect [38-41]. Therefore, excessive intake of seaweed and iodine or not requires further recommendations.
- It has been reported that the expression level of SLC5A5 has been reported to be lower in thyroid tumor tissues than that in normal tissues in Korean and Italian subjects [42,43]. Although SLC5A5 plays a crucial role in iodine metabolism and thyroid regulation, the relationship between SLC5A5 and thyroid cancer is complex and poorly understood [15,44]. Russo and collaborators found that mutations as well as other genetic alterations of SLC5A5 may not be the main cause of reduced iodine uptake in differentiated thyroid carcinomas [44,45]. However, in the present study, our findings suggested that SLC5A5 rs77277498 polymorphism may enhance the potential protective effect of dietary iodine-based intake on thyroid cancer. Nevertheless, the results regarding the methylation of the SLC5A5 promoter in thyroid cancer are still controversial, and thus, further studies on the role of SLC5A5 in thyroid cancer are warranted [44].
- To the authors’ knowledge, no study has yet elucidated the effect modification of SLC5A5 on the associations between dietary seaweed and iodine intakes and thyroid cancer. In this study, a comprehensive and validated FFQ was administered by trained interviewers to acquire dietary information from the subjects [19,46-48]. Moreover, iodine and iodine-containing food consumption was estimated by residual energy adjustment, which has been suggested to be more powerful and robust to residual confounding than the standard method [21] and to diminish the effect of misreporting of energy and micronutrient intake [49]. Furthermore, by using the largely imputed data of approximately 5 million tags, this study not only determined the association between dietary iodine consumption and thyroid cancer risk but also identified how this association varies according to different genotypes of SLC5A5 polymorphisms.
- Nevertheless, some limitations need to be considered when interpreting the present results. First, the presence of selection and recall biases related to a hospital-based case-control study should be mentioned. In addition, the associations between dietary intake and thyroid cancer could occur without a causal relationship because patients who are aware of their disease status might focus on healthy dietary behaviors [50,51], or because of the increased detection of early cases due to the thyroid cancer screening program available in Korea [52,53]. Second, the sample size was relatively small for the subgroup analyses by tumor subtype and tumor aggressiveness. However, our previous studies reported that most of the cases (97%) in our study population were papillary thyroid cancer [54]. Additionally, according to the Korea National Cancer Incidence Database, papillary carcinoma was reported to be the most frequent histologic type (94.2%) among incident thyroid cancer cases in Korea during 1997 and 2011 [55,56]. The results therefore provide supportive evidence for papillary thyroid cancer but not for other subtypes, such as differentiated or follicular thyroid cancer. The relatively small sample size might also not represent the consumption by Korea adults. However, by using dietary data from the total Cancer Screenee Cohort of 10,810 participants [50], our estimates of iodine and iodine-based food consumption in the present study did not differ from those that were not included in the final analysis (Supplemental Table S3). Third, due to the data unavailability, we were unable to examine or adjust for the effect of previous radiation exposure, which is a strong risk factor for thyroid cancer. Finally, because of the lack of relevant items on the SQFFQ, we could not investigate the effect of all items of edible seaweed and their bioactive components, only the three most commonly consumed types of seaweed in Korea [57,58]. Thus, the SQFFQ may therefore underestimate the actual amount of iodine intake in the Korean population.
- In summary, our findings provide evidence to support the putative effects of dietary gim and iodine intakes against thyroid cancer risk, and the strength of this association can be modified by rs77277498 genotypes.
DISCUSSION
Supplementary Information
Supplemental Table S1.
Supplemental Table S2.
Supplemental Table S3.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: T.H., E.K.L., J.L., Y.H., J.K. Acquisition, analysis, or interpretation of data: T.H., E.K.L., J.K. Drafting the work or revising: T.H., E.K.L., J.K. Final approval of the manuscript: T.H., E.K.L., J.L., Y.H., J.K.
Article information
-
Acknowledgements
- This study was funded by grants from the National Cancer Center in Korea (No. 2210990), National Research Foundation funded by the South Korean Government (2018R1D1A1A09083876), and Patient-Centered Clinical Research Coordinating Center funded by the Ministry of Health & Welfare, Republic of Korea (No. HI19C0481, HC19C013).
- The author, Tung Hoang, expresses appreciation for the support from the Global Leadership program of the Korea Safety Health.
| Demographic | Cases (n=117) | Controls (n=173) | P value |
|---|---|---|---|
| Age, yr | 52.5±8.0 | 51.7±8.1 | 0.417 |
| Age group, yr | 0.334 | ||
| <39 | 1 (0.9) | 8 (4.6) | |
| 40–49 | 44 (37.6) | 65 (37.6) | |
| 50–59 | 45 (38.5) | 64 (37.0) | |
| >60 | 27 (23.1) | 36 (20.8) | |
| Sex | 0.001a | ||
| Male | 6 (5.1) | 33 (19.1) | |
| Female | 111 (94.9) | 140 (80.9) | |
| Family history of thyroid cancer | 0.216 | ||
| No | 104 (88.9) | 163 (94.2) | |
| Yes | 9 (7.7) | 8 (4.6) | |
| Missing | 4 (3.4) | 2 (1.2) | |
| Marital status | 0.189 | ||
| Married | 97 (82.9) | 147 (85.0) | |
| Other | 16 (13.7) | 25 (14.5) | |
| Missing | 4 (3.4) | 1 (0.6) | |
| Educational level | 0.076 | ||
| ≤Middle school | 21 (17.9) | 24 (13.9) | |
| High school | 36 (30.8) | 78 (45.1) | |
| ≥College | 53 (45.3) | 66 (38.2) | |
| Missing | 7 (6.0) | 5 (2.9) | |
| Occupation | 0.137 | ||
| Housewives, profession, and office worker | 88 (75.2) | 109 (63.0) | |
| Sales, service | 14 (12.0) | 35 (20.2) | |
| Agriculture, laborer, unemployed, and others | 13 (11.1) | 27 (15.6) | |
| Missing | 2 (1.7) | 2 (1.2) | |
| Monthly income, $ | 0.322 | ||
| <1,667 | 26 (22.2) | 37 (21.4) | |
| 1,667–3,333 | 36 (30.8) | 60 (34.7) | |
| >3,333 | 36 (30.8) | 60 (34.7) | |
| Missing | 19 (16.2) | 16 (9.2) | |
| Smoking status | 0.016a | ||
| Never | 108 (92.3) | 139 (80.3) | |
| Ever | 9 (7.7) | 32 (18.5) | |
| Missing | 0 | 2 (1.2) | |
| Alcohol consumption | 0.166 | ||
| Never | 72 (61.5) | 91 (52.6) | |
| Ever | 45 (38.5) | 82 (47.4) | |
| Regular exercise | 0.984 | ||
| Yes | 47 (40.2) | 70 (40.5) | |
| No | 39 (33.3) | 56 (32.4) | |
| Missing | 31 (26.5) | 47 (27.2) | |
| BMI, kg/m2 | 23.6±3.0 | 23.4±2.8 | 0.497 |
| BMI group, kg/m2 | 0.144 | ||
| <23 | 48 (41.0) | 82 (47.4) | |
| 23–24.9 | 37 (31.6) | 36 (20.8) | |
| ≥25 | 31 (26.5) | 50 (28.9) | |
| Missing | 1 (0.9) | 5 (2.9) |
| Factor | Cases (n=117) | Controls (n=173) | P value |
|---|---|---|---|
| Gim | |||
| Amount, g/day | 0.9±1.4 | 1.0±1.3 | 0.497 |
| Low (≤0.611) | 69 (59.0) | 76 (43.9) | 0.017a |
| High (>0.611) | 48 (41.0) | 97 (56.1) | |
| Frequency (times) | |||
| ≤3, /mo | 34 (29.1) | 50 (28.9) | 0.559 |
| 1–4, /wk | 58 (49.6) | 77 (44.5) | |
| Almost daily | 25 (21.4) | 46 (26.6) | |
| Miyeok/dashima | |||
| Amount, g/day | 0.9±1.3 | 1.0±1.7 | 0.406 |
| Low (≤0.564) | 60 (51.3) | 85 (49.1) | 0.811 |
| High (>0.564) | 57 (48.7) | 88 (50.9) | |
| Frequency (times) | |||
| ≤3, /mo | 71 (60.7) | 102 (59.0) | 0.912 |
| 1–4, /wk | 38 (32.5) | 57 (32.9) | |
| Almost daily | 8 (6.8) | 14 (8.1) | |
| Total seaweed, g/day | 1.7±2.0 | 2.0±2.4 | 0.332 |
| Low (≤1.33) | 63 (53.8) | 82 (47.4) | 0.338 |
| High (>1.33) | 54 (46.2) | 91 (52.6) | |
| Iodine, μg/day | 626.4±539.8 | 629.1±506.5 | 0.966 |
| Low (≤523) | 64 (54.7) | 81 (46.8) | 0.231 |
| High (>523) | 53 (45.3) | 92 (53.2) | |
| rs77277498 (C>G) | |||
| C/C | 95 (81.2) | 118 (68.2) | 0.027a |
| G/C | 22 (18.8) | 50 (28.9) | |
| G/G | 0 | 3 (1.7) | |
| Missing | 0 | 2 (1.2) |
| Factor | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Gim | ||
| Amount, g/day | ||
| Low (≤0.611) | 1 (reference) | 1 (reference) |
| High (>0.611) | 0.55 (0.34–0.87)a | 0.50 (0.30–0.83)a |
| Frequency (times) | ||
| ≤3, /mo | 1 (reference) | 1 (reference) |
| 1–4, /wk | 1.11 (0.64–1.93) | 1.20 (0.66–2.18) |
| Almost daily | 0.80 (0.41–1.53) | 0.69 (0.34–1.39) |
| Miyeok/dashima | ||
| Amount, g/day | ||
| Low (≤0.564) | 1 (reference) | 1 (reference) |
| High (>0.564) | 0.92 (0.57–1.47) | 0.74 (0.44–1.23) |
| Frequency (times) | ||
| ≤3, /mo | 1 (reference) | 1 (reference) |
| 1–4, /wk | 0.96 (0.57–1.59) | 0.82 (0.47–1.41) |
| Almost daily | 0.82 (0.31–2.02) | 0.60 (0.22–1.57) |
| Total seaweed, g/day | ||
| Low (≤1.33) | 1 (reference) | 1 (reference) |
| High (>1.33) | 0.77 (0.48–1.24) | 0.61 (0.36–1.02) |
| Iodine, μg/day | ||
| Low (≤523) | 1 (reference) | 1 (reference) |
| High (>523) | 0.73 (0.45–1.17) | 0.57 (0.33–0.95)a |
| rs77277498 (C>G) | ||
| C/C | 1 (reference) | 1 (reference) |
| G/C+G/G | 0.52 (0.29–0.90)a | 0.52 (0.28–0.93)a |
| Dietary factor |
Crude OR (95% CI) |
Adjusted OR (95% CI) |
||||
|---|---|---|---|---|---|---|
| C/C | G/G+G/C | Pint | C/C | G/G+G/C | Pint | |
| Gim | ||||||
| Amount, g/day | ||||||
| Low (≤0.611) | 1 (reference) | 0.81 (0.35–1.80) | 0.199 | 1 (reference) | 0.78 (0.32–1.85) | 0.278 |
| High (>0.611) | 0.70 (0.40–1.20) | 0.26 (0.11–0.58)a | 0.63 (0.35–1.13) | 0.25 (0.10–0.56)a | ||
| Frequency (times) | ||||||
| ≤3, /mo | 1 (reference) | 0.21 (0.04–0.69)a | 0.044 | 1 (reference) | 0.48 (0.23–0.99)a | 0.020a |
| 1–4, /wk | 0.82 (0.44–1.53) | 0.90 (0.38–2.13) | 0.93 (0.51–1.69) | 0.56 (0.23–1.31) | ||
| Almost daily | 0.88 (0.41–1.88) | 0.27 (0.08–0.77)a | 0.79 (0.29–2.05) | - | ||
| Miyeok/dashima | ||||||
| Amount, g/day | ||||||
| Low (≤0.564) | 1 (reference) | 0.65 (0.30–1.37) | 0.347 | 1 (reference) | 0.65 (0.29–1.45) | 0.365 |
| High (>0.564) | 1.03 (0.60–1.77) | 0.39 (0.15–0.90)a | 0.84 (0.46–1.51) | 0.31 (0.12–0.77)a | ||
| Frequency (times) | ||||||
| ≤3, /mo | 1 (reference) | 0.19 (0.04–0.70)a | 0.531 | 1 (reference) | 0.50 (0.22–1.08) | 0.483 |
| 1–4, /wk | 0.83 (0.42–1.63) | 1.11 (0.43–2.87) | 0.81 (0.42–1.53) | 0.47 (0.18–1.15) | ||
| Almost daily | 0.80 (0.34–1.84) | 0.22 (0.06–0.66)a | 0.60 (0.21–1.65) | - | ||
| Total seaweed, g/day | ||||||
| Low (≤1.33) | 1 (reference) | 0.83 (0.38–1.79) | 0.092 | 1 (reference) | 0.78 (0.33–1.77) | 0.180 |
| High (>1.33) | 1.00 (0.58–1.73) | 0.31 (0.12–0.71)a | 0.77 (0.43–1.39) | 0.26 (0.10–0.62)a | ||
| Iodine, μg/day | ||||||
| Low (≤523) | 1 (reference) | 0.43 (0.20–0.91)a | 0.587 | 1 (reference) | 0.42 (0.18–0.93)a | 0.570 |
| High (>523) | 0.66 (0.38–1.13) | 0.39 (0.16–0.90)a | 0.51 (0.27–0.93)a | 0.30 (0.12–0.73)a | ||
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49.ArticlePubMedPDF
- 2. Jung KW, Won YJ, Hong S, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2021. Cancer Res Treat 2021;53:316–22.ArticlePubMedPMCPDF
- 3. Iglesias ML, Schmidt A, Ghuzlan AA, Lacroix L, Vathaire F, Chevillard S, et al. Radiation exposure and thyroid cancer: a review. Arch Endocrinol Metab 2017;61:180–7.ArticlePubMedPMC
- 4. Kitahara CM, McCullough ML, Franceschi S, Rinaldi S, Wolk A, Neta G, et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid 2016;26:306–18.ArticlePubMedPMC
- 5. Aakre I, Tveito Evensen L, Kjellevold M, Dahl L, Henjum S, Alexander J, et al. Iodine status and thyroid function in a group of seaweed consumers in Norway. Nutrients 2020;12:3483.ArticlePubMedPMC
- 6. Ju DL, Park YJ, Paik HY, Kim MJ, Park S, Jung KY, et al. Dietary evaluation of a low-iodine diet in Korean thyroid cancer patients preparing for radioactive iodine therapy in an iodine-rich region. Nutr Res Pract 2016;10:167–74.ArticlePubMedPMCPDF
- 7. Han MR, Ju DL, Park YJ, Paik HY, Song Y. An iodine database for common Korean foods and the association between iodine intake and thyroid disease in Korean adults. Int J Thyroidol 2015;8:170–82.Article
- 8. Park JK, Woo HW, Kim MK, Shin J, Lee YH, Shin DH, et al. Dietary iodine, seaweed consumption, and incidence risk of metabolic syndrome among postmenopausal women: a prospective analysis of the Korean Multi-Rural Communities Cohort Study (MRCohort). Eur J Nutr 2021;60:135–46.ArticlePubMedPDF
- 9. Kim J, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, et al. Associations among dietary seaweed intake, c-MYC rs698-3267 polymorphism, and risk of colorectal cancer in a Korean population: a case-control study. Eur J Nutr 2020;59:1963–74.ArticlePubMedPDF
- 10. Ganesa AR, Tiwari U, Rajauria G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci Hum Wellness 2019;8:252–63.Article
- 11. Ruan BF, Ge WW, Lin MX, Li QS. A review of the components of seaweeds as potential candidates in cancer therapy. Anticancer Agents Med Chem 2018;18:354–66.ArticlePubMedPDF
- 12. Gutierrez-Rodriguez AG, Juarez-Portilla C, Olivares-Banuelos T, Zepeda RC. Anticancer activity of seaweeds. Drug Discov Today 2018;23:434–47.ArticlePubMed
- 13. de Morais RM, Sobrinho AB, de Souza Silva CM, de Oliveira JR, da Silva I, de Toledo Nobrega O. The role of the NIS (SLC5A5) gene in papillary thyroid cancer: a systematic review. Int J Endocrinol 2018;2018:9128754.PubMedPMC
- 14. Tavares C, Coelho MJ, Eloy C, Melo M, da Rocha AG, Pestana A, et al. NIS expression in thyroid tumors, relation with prognosis clinicopathological and molecular features. Endocr Connect 2018;7:78–90.ArticlePubMedPMC
- 15. Darrouzet E, Lindenthal S, Marcellin D, Pellequer JL, Pourcher T. The sodium/iodide symporter: state of the art of its molecular characterization. Biochim Biophys Acta 2014;1838(1 Pt B):244–53.ArticlePubMed
- 16. Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer 2006;13:797–826.ArticlePubMed
- 17. Kim J. Cancer screenee cohort study of the National Cancer Center in South Korea. Epidemiol Health 2014;36:e2014013.ArticlePubMedPMC
- 18. Hoang T, Nguyen Ngoc Q, Lee J, Lee EK, Hwangbo Y, Kim J. Evaluation of modifiable factors and polygenic risk score in thyroid cancer. Endocr Relat Cancer 2021;28:481–94.ArticlePubMed
- 19. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr 2007;61:1435–41.ArticlePubMedPDF
- 20. Tomova GD, Arnold KF, Gilthorpe MS, Tennant P. Adjustment for energy intake in nutritional research: a causal inference perspective. Am J Clin Nutr 2022;115:189–98.ArticlePubMed
- 21. Brown CC, Kipnis V, Freedman LS, Hartman AM, Schatzkin A, Wacholder S. Energy adjustment methods for nutritional epidemiology: the effect of categorization. Am J Epidemiol 1994;139:323–38.ArticlePubMed
- 22. Delaneau O, Howie B, Cox AJ, Zagury JF, Marchini J. Haplotype estimation using sequencing reads. Am J Hum Genet 2013;93:687–96.ArticlePubMedPMC
- 23. Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009;5:e1000529.ArticlePubMedPMC
- 23. Cho TJ, Rhee MS. Health functionality and quality control of laver (Porphyra, Pyropia): current issues and future perspectives as an edible seaweed. Mar Drugs 2019;18:14.ArticlePubMedPMC
- 25. Etman SM, Abdallah OY, Elnaggar YSR. Novel fucoidan based bioactive targeted nanoparticles from Undaria pinnatifida for treatment of pancreatic cancer. Int J Biol Macromol 2020;145:390–401.ArticlePubMed
- 26. Hsu HY, Hwang PA. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin Transl Med 2019;8:15.ArticlePubMedPMCPDF
- 27. Burney M, Mathew L, Gaikwad A, Nugent EK, Gonzalez AO, Smith JA. Evaluation fucoidan extracts from Undaria pinnatifida and Fucus vesiculosus in combination with anticancer drugs in human cancer orthotopic mouse models. Integr Cancer Ther 2018;17:755–61.ArticlePubMedPMCPDF
- 28. Han YS, Lee JH, Lee SH. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol Med Rep 2015;12:3446–52.ArticlePubMedPMC
- 29. Mak W, Wang SK, Liu T, Hamid N, Li Y, Lu J, et al. Anti-proliferation potential and content of fucoidan extracted from sporophyll of New Zealand Undaria pinnatifida. Front Nutr 2014;1:9.ArticlePubMedPMC
- 30. Yang L, Wang P, Wang H, Li Q, Teng H, Liu Z, et al. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar Drugs 2013;11:1961–76.ArticlePubMedPMC
- 31. Tian L, Li CM, Li YF, Huang TM, Chao NX, Luo GR, et al. Laminarin from seaweed (Laminaria japonica) inhibits hepatocellular carcinoma through upregulating senescence marker protein-30. Cancer Biother Radiopharm 2020;35:277–83.ArticlePubMedPMC
- 32. Mei C, Zhou S, Zhu L, Ming J, Zeng F, Xu R. Antitumor effects of Laminaria extract fucoxanthin on lung cancer. Mar Drugs 2017;15:39.ArticlePubMedPMC
- 33. Go H, Hwang HJ, Nam TJ. A glycoprotein from Laminaria japonica induces apoptosis in HT-29 colon cancer cells. Toxicol In Vitro 2010;24:1546–53.ArticlePubMed
- 34. Yang H, Zeng M, Dong S, Liu Z, Li R. Anti-proliferative activity of phlorotannin extracts from brown algae Laminaria japonica Aresch. Chin J Oceanol Limnol 2010;28:122–30.ArticlePDF
- 35. Yao M, Qian X, Qin H. Effects of Laminaria japonica polysaccharides on the survival of non-small-cell lung cancer A549 cells. Int J Polym Sci 2019;2019:1–9.ArticlePDF
- 36. Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res 2015;8:8.ArticlePubMedPMCPDF
- 37. Cao LZ, Peng XD, Xie JP, Yang FH, Wen HL, Li S. The relationship between iodine intake and the risk of thyroid cancer: a meta-analysis. Medicine (Baltimore) 2017;96:e6734.PubMedPMC
- 38. Farebrother J, Zimmermann MB, Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Ann N Y Acad Sci 2019;1446:44–65.ArticlePubMedPDF
- 39. Katagiri R, Yuan X, Kobayashi S, Sasaki S. Effect of excess iodine intake on thyroid diseases in different populations: a systematic review and meta-analyses including observational studies. PLoS One 2017;12:e0173722.ArticlePubMedPMC
- 40. Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol 2014;10:136–42.ArticlePubMedPMCPDF
- 41. Kim K, Cho SW, Park YJ, Lee KE, Lee DW, Park SK. Association between iodine intake, thyroid function, and papillary thyroid cancer: a case-control study. Endocrinol Metab (Seoul) 2021;36:790–9.ArticlePubMedPMCPDF
- 42. Rosignolo F, Maggisano V, Sponziello M, Celano M, Di Gioia CR, D’Agostino M, et al. Reduced expression of THRβ in papillary thyroid carcinomas: relationship with BRAF mutation, aggressiveness and miR expression. J Endocrinol Invest 2015;38:1283–9.ArticlePubMedPDF
- 43. Lee SJ, Choi KC, Han JP, Park YE, Choi MG. Relationship of sodium/iodide symporter expression with I131 whole body scan uptake between primary and metastatic lymph node papillary thyroid carcinomas. J Endocrinol Invest 2007;30:28–34.ArticlePubMedPDF
- 44. Zafon C, Gil J, Perez-Gonzalez B, Jorda M. DNA methylation in thyroid cancer. Endocr Relat Cancer 2019;26:R415–39.ArticlePubMed
- 45. Russo D, Manole D, Arturi F, Suarez HG, Schlumberger M, Filetti S, et al. Absence of sodium/iodide symporter gene mutations in differentiated human thyroid carcinomas. Thyroid 2001;11:37–9.ArticlePubMed
- 46. Kim JH, Lee J, Choi IJ, Kim YI, Kim J. Dietary patterns and gastric cancer risk in a Korean population: a case-control study. Eur J Nutr 2021;60:389–97.ArticlePubMedPDF
- 47. Gunathilake M, Lee J, Choi IJ, Kim YI, Kim J. identification of dietary pattern networks associated with gastric cancer using gaussian graphical models: a case-control study. Cancers (Basel) 2020;12:1044.ArticlePubMedPMC
- 48. Kim JH, Lee J, Choi IJ, Kim YI, Kwon O, Kim H, et al. Dietary carotenoids intake and the risk of gastric cancer: a case-control study in Korea. Nutrients 2018;10:1031.ArticlePubMedPMC
- 49. Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van’t Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr 2009;101 Suppl 2:S73–85.ArticlePubMed
- 50. Hoang T, Lee J, Kim J. Differences in dietary patterns identified by the gaussian graphical model in Korean adults with and without a self-reported cancer diagnosis. J Acad Nutr Diet 2021;121:1484–96.ArticlePubMed
- 51. Hoang T, Lee J, Kim J, Park B. Food intake behavior in cancer survivors in comparison with healthy general population; from the health examination center-based cohort. J Cancer Prev 2019;24:208–16.ArticlePubMedPMC
- 52. Jung YS, Oh CM, Kim Y, Jung KW, Ryu J, Won YJ. Longterm survival of patients with thyroid cancer according to the methods of tumor detection: a nationwide cohort study in Korea. PLoS One 2018;13:e0194743.ArticlePubMedPMC
- 53. Ahn HS, Kim HJ, Kim KH, Lee YS, Han SJ, Kim Y, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid 2016;26:1535–40.ArticlePubMed
- 54. Hoang T, Song D, Lee J, Lee EK, Hwangbo Y, Kim J. Association among body mass index, genetic variants of FTO, and thyroid cancer risk: a hospital-based case-control study of the Cancer Screenee Cohort in Korea. Cancer Res Treat 2021;53:857–73.ArticlePubMedPMCPDF
- 55. Myung SK, Lee CW, Lee J, Kim J, Kim HS. Risk factors for thyroid cancer: a hospital-based case-control study in Korean adults. Cancer Res Treat 2017;49:70–8.ArticlePubMedPMCPDF
- 56. Oh CM, Jung KW, Won YJ, Shin A, Kong HJ, Lee JS. Age-period-cohort analysis of thyroid cancer incidence in Korea. Cancer Res Treat 2015;47:362–9.ArticlePubMedPMCPDF
- 57. Cherry P, O’Hara C, Magee PJ, McSorley EM, Allsopp PJ. Risks and benefits of consuming edible seaweeds. Nutr Rev 2019;77:307–29.ArticlePubMedPMC
- 58. Penalver R, Lorenzo JM, Ros G, Amarowicz R, Pateiro M, Nieto G. Seaweeds as a functional ingredient for a healthy diet. Mar Drugs 2020;18:301.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Iodine nutrition and papillary thyroid cancer
Xueqi Zhang, Fan Zhang, Qiuxian Li, Chuyao Feng, Weiping Teng
Frontiers in Nutrition.2022;[Epub] CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite