Thyroid Cancer Screening
- Page Path
- HOME > BROWSE ARTICLES > Thyroid Cancer Screening
Original Articles
- Thyroid
Thyroid Cancer Screening - Diagnostic Performance of Ultrasound-Based Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Meta-Analysis
- Leehi Joo, Min Kyoung Lee, Ji Ye Lee, Eun Ju Ha, Dong Gyu Na
- Endocrinol Metab. 2023;38(1):117-128. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1670

- 2,201 View
- 166 Download
- 3 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
This study investigated the diagnostic performance of biopsy criteria in four society ultrasonography risk stratification systems (RSSs) for thyroid nodules, including the 2021 Korean (K)-Thyroid Imaging Reporting and Data System (TIRADS).
Methods
The Ovid-MEDLINE, Embase, Cochrane, and KoreaMed databases were searched and a manual search was conducted to identify original articles investigating the diagnostic performance of biopsy criteria for thyroid nodules (≥1 cm) in four widely used society RSSs.
Results
Eleven articles were included. The pooled sensitivity and specificity were 82% (95% confidence interval [CI], 74% to 87%) and 60% (95% CI, 52% to 67%) for the American College of Radiology (ACR)-TIRADS, 89% (95% CI, 85% to 93%) and 34% (95% CI, 26% to 42%) for the American Thyroid Association (ATA) system, 88% (95% CI, 81% to 92%) and 42% (95% CI, 22% to 67%) for the European (EU)-TIRADS, and 96% (95% CI, 94% to 97%) and 21% (95% CI, 17% to 25%) for the 2016 K-TIRADS. The sensitivity and specificity were 76% (95% CI, 74% to 79%) and 50% (95% CI, 49% to 52%) for the 2021 K-TIRADS1.5 (1.5-cm size cut-off for intermediate-suspicion nodules). The pooled unnecessary biopsy rates of the ACR-TIRADS, ATA system, EU-TIRADS, and 2016 K-TIRADS were 41% (95% CI, 32% to 49%), 65% (95% CI, 56% to 74%), 68% (95% CI, 60% to 75%), and 79% (95% CI, 74% to 83%), respectively. The unnecessary biopsy rate was 50% (95% CI, 47% to 53%) for the 2021 K-TIRADS1.5.
Conclusion
The unnecessary biopsy rate of the 2021 K-TIRADS1.5 was substantially lower than that of the 2016 K-TIRADS and comparable to that of the ACR-TIRADS. The 2021 K-TIRADS may help reduce potential harm due to unnecessary biopsies. -
Citations
Citations to this article as recorded by- To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinology and Metabolism.2023; 38(1): 72. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef - 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules
Young Joo Park, Eun Kyung Lee, Young Shin Song, Soo Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung
International Journal of Thyroidology.2023; 16(1): 1. CrossRef - Evaluation of the Appropriateness of Thyroid Fine-Needle Aspiration
Lairce Cristina Ribeiro Brito, Iara Beatriz De Carvalho Botêlho, Lanna Matos Silva Fernandes, Nayze Lucena Sangreman Aldeman, Uziel Nunes Silva
International Journal for Innovation Education and Research.2023; 11(6): 8. CrossRef
- To Screen or Not to Screen?

- Thyroid
Thyroid Cancer Screening - A Comprehensive Assessment of the Harms of Fine-Needle Aspiration Biopsy for Thyroid Nodules: A Systematic Review
- Ji Yong Park, Wonsuk Choi, A Ram Hong, Jee Hee Yoon, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2023;38(1):104-116. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1669
- 3,584 View
- 160 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
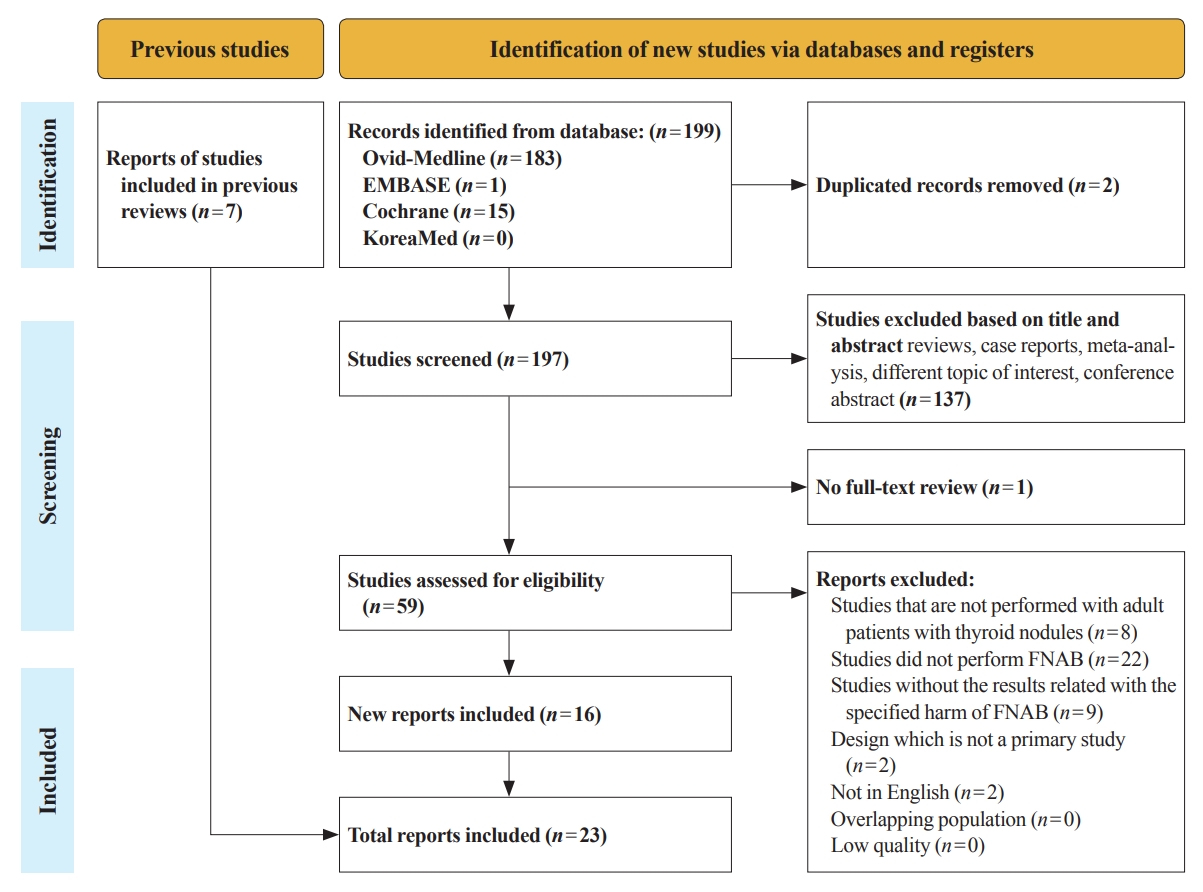
There have concerns related with the potential harms of fine-needle aspiration biopsy (FNAB). We aimed to summarize the clinical complications and evaluate the safety of FNAB.
Methods
Studies related with the harms of FNAB were searched on MEDLINE, Embase, Cochrane library, and KoreaMed from 2012 to 2022. Also, studies reviewed in the previous systematic reviews were evaluated. Included clinical complications were postprocedural pain, bleeding events, neurological symptoms, tracheal puncture, infections, post-FNAB thyrotoxicosis, and needle tract implantation of thyroid cancers.
Results
Twenty-three cohort studies were included in this review. Nine studies which were related with FNAB-related pain showed that most of the subjects had no or mild discomfort. The 0% to 6.4% of the patients had hematoma or hemorrhage after FNAB, according to 15 studies. Vasovagal reaction, vocal cord palsy, and tracheal puncture have rarely described in the included studies. Needle tract implantation of thyroid malignancies was described in three studies reporting 0.02% to 0.19% of the incidence rate.
Conclusion
FNAB is considered to be a safe diagnostic procedure with rare complications, which are mainly minor events. Thorough assessement of the patients’ medical condition when deciding to perform FNABs would be advisable to lower potential complications. -
Citations
Citations to this article as recorded by- A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules
Eun Kyung Lee, Young Joo Park, Chan Kwon Jung, Dong Gyu Na
Endocrinology and Metabolism.2024; 39(1): 61. CrossRef - Fine-needle aspiration cytology for neck lesions in patients with antithrombotic/anticoagulant medications: systematic review and meta-analysis
Dongbin Ahn, Ji Hye Kwak, Gill Joon Lee, Jin Ho Sohn
European Radiology.2024;[Epub] CrossRef - To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef - Evaluation of the Appropriateness of Thyroid Fine-Needle Aspiration
Lairce Cristina Ribeiro Brito, Iara Beatriz De Carvalho Botêlho, Lanna Matos Silva Fernandes, Nayze Lucena Sangreman Aldeman, Uziel Nunes Silva
International Journal for Innovation Education and Research.2023; 11(6): 8. CrossRef
- A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules

- Thyroid
Thyroid Cancer Screening - Lower Thyroid Cancer Mortality in Patients Detected by Screening: A Meta-Analysis
- Shinje Moon, Young Shin Song, Kyong Yeun Jung, Eun Kyung Lee, Young Joo Park
- Endocrinol Metab. 2023;38(1):93-103. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1667
- 2,164 View
- 116 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Thyroid cancer screening has contributed to the skyrocketing prevalence of thyroid cancer. However, the true benefit of thyroid cancer screening is not fully understood. This study aimed to evaluate the impact of screening on the clinical outcomes of thyroid cancer by comparing incidental thyroid cancer (ITC) with non-incidental thyroid cancer (NITC) through a meta-analysis.
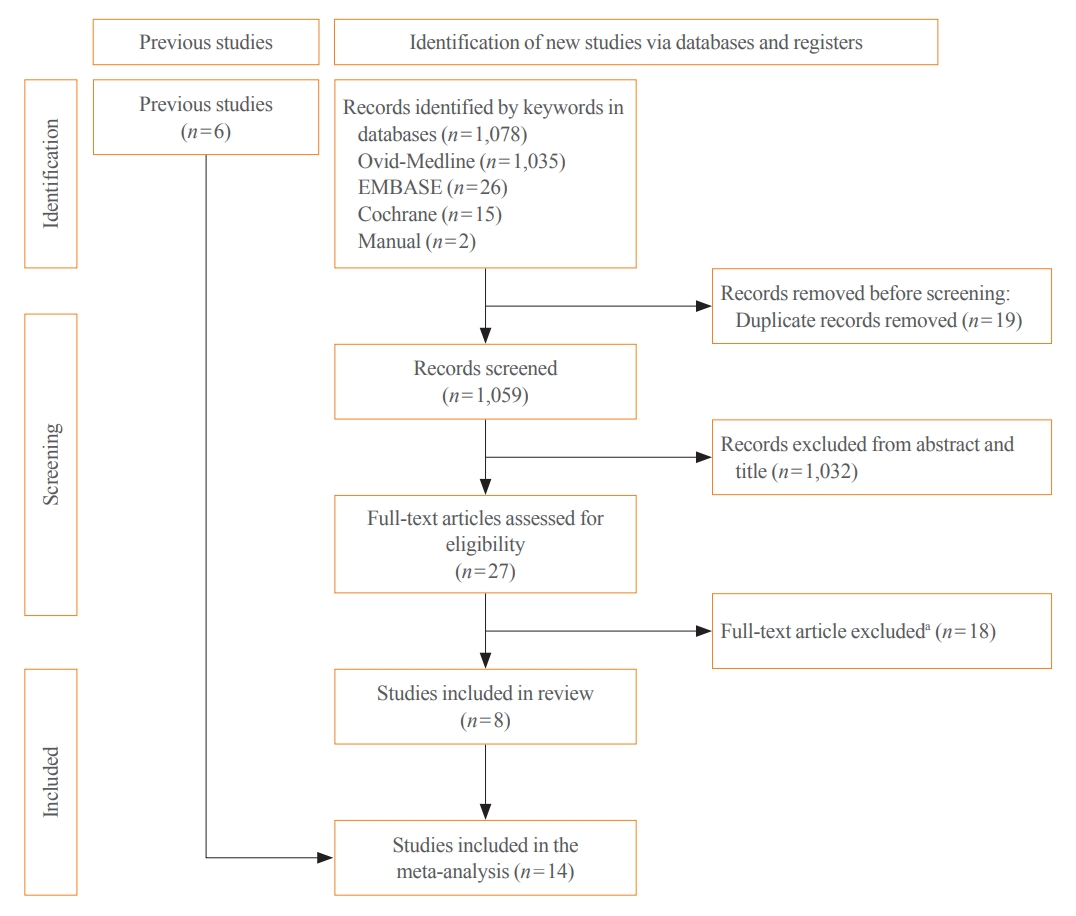
Methods
PubMed and Embase were searched from inception to September 2022. We estimated and compared the prevalence of high-risk features (aggressive histology of thyroid cancer, extrathyroidal extension, metastasis to regional lymph nodes or distant organs, and advanced tumor-node-metastasis [TNM] stage), thyroid cancer-specific death, and recurrence in the ITC and NITC groups. We also calculated pooled risks and 95% confidence intervals (CIs) of the outcomes derived from these two groups.
Results
From 1,078 studies screened, 14 were included. In comparison to NITC, the ITC group had a lower incidence of aggressive histology (odds ratio [OR], 0.46; 95% CI, 0.31 to 0.7), smaller tumors (mean difference, −7.9 mm; 95% CI, −10.2 to −5.6), lymph node metastasis (OR, 0.64; 95% CI, 0.48 to 0.86), and distant metastasis (OR, 0.42; 95% CI, 0.23 to 0.77). The risks of recurrence and thyroid cancer-specific mortality were also lower in the ITC group (OR, 0.42; 95% CI, 0.25 to 0.71 and OR, 0.46; 95% CI, 0.28 to 0.74) than in the NITC group.
Conclusion
Our findings provide important evidence of a survival benefit from the early detection of thyroid cancer compared to symptomatic thyroid cancer. -
Citations
Citations to this article as recorded by- To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinology and Metabolism.2023; 38(1): 72. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef - Delayed Surgery for and Outcomes of Papillary Thyroid Cancer: Is the Pendulum Still Swinging?
Giorgio Grani
Clinical Thyroidology.2023; 35(5): 192. CrossRef
- To Screen or Not to Screen?

- Thyroid
Thyroid Cancer Screening - Survival Comparison of Incidentally Found versus Clinically Detected Thyroid Cancers: An Analysis of a Nationwide Cohort Study
- Shinje Moon, Eun Kyung Lee, Hoonsung Choi, Sue K. Park, Young Joo Park
- Endocrinol Metab. 2023;38(1):81-92. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1668

- 1,714 View
- 153 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The true benefit of thyroid cancer screening is incompletely understood. This study investigated the impact of ultrasound screening on thyroid cancer outcomes through a comparison with symptomatic thyroid cancer using data from a nationwide cohort study in Korea.
Methods
Cox regression analysis was performed to assess the hazard ratios (HRs) for all-cause and thyroid cancer-specific mortality. Considering the possible bias arising from age, sex, year of thyroid cancer registration, and confounding factors for mortality (including smoking/drinking status, diabetes, and hypertension), all analyses were conducted with stabilized inverse probability of treatment weighting (IPTW) according to the route of detection.
Results
Of 5,796 patients with thyroid cancer, 4,145 were included and 1,651 were excluded due to insufficient data. In comparison with the screening group, the clinical suspicion group was associated with large tumors (17.2±14.6 mm vs. 10.4±7.9 mm), advanced T stage (3–4) (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.09 to 1.41), extrathyroidal extension (OR, 1.16; 95% CI, 1.02 to 1.32), and advanced stage (III–IV) (OR, 1.16; 95% CI, 1.00 to 1.35). In IPTW-adjusted Cox regression analysis, the clinical suspicion group had significantly higher risks of all-cause mortality (HR, 1.43; 95% CI, 1.14 to 1.80) and thyroid cancer-specific mortality (HR, 3.07; 95% CI, 1.77 to 5.29). Mediation analysis showed that the presence of thyroid-specific symptoms was directly associated with a higher risk of cancer-specific mortality. Thyroid-specific symptoms also indirectly affected thyroid cancer-specific mortality, mediated by tumor size and advanced clinicopathologic status.
Conclusion
Our findings provide important evidence for the survival benefit of early detection of thyroid cancer compared to symptomatic thyroid cancer. -
Citations
Citations to this article as recorded by- Clinical Characteristics, Diagnostic Approach and Outcome of Thyroid Incidental Findings vs. Clinically Overt Thyroid Nodules: An Observational Single-Centre Study
Tom Jansen, Nike Stikkelbroeck, Annenienke van de Ven, Ilse van Engen-van Grunsven, Marcel Janssen, Han Bonenkamp, Martin Gotthardt, Romana T. Netea-Maier
Cancers.2023; 15(8): 2350. CrossRef - Lower Thyroid Cancer Mortality in Patients Detected by Screening: A Meta-Analysis
Shinje Moon, Young Shin Song, Kyong Yeun Jung, Eun Kyung Lee, Young Joo Park
Endocrinology and Metabolism.2023; 38(1): 93. CrossRef - To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinology and Metabolism.2023; 38(1): 72. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef
- Clinical Characteristics, Diagnostic Approach and Outcome of Thyroid Incidental Findings vs. Clinically Overt Thyroid Nodules: An Observational Single-Centre Study

Editorials
- Thyroid
Thyroid Cancer Screening - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
- Jung Hwan Baek
- Endocrinol Metab. 2023;38(1):75-77. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.105
- 1,243 View
- 111 Download
- 1 Web of Science
- 1 Crossref

- Thyroid
Thyroid Cancer Screening - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
- Ka Hee Yi
- Endocrinol Metab. 2023;38(1):72-74. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.106
- 1,019 View
- 81 Download

- Thyroid
Thyroid Cancer Screening - To Screen or Not to Screen?
- Do Joon Park
- Endocrinol Metab. 2023;38(1):69-71. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.104
- 907 View
- 94 Download


 KES
KES

 First
First Prev
Prev



