Search
- Page Path
- HOME > Search
- Risk of Subsequent Primary Cancers in Thyroid Cancer Survivors according to the Dose of Levothyroxine: A Nationwide Cohort Study
- Min-Su Kim, Jang Won Lee, Min Kyung Hyun, Young Shin Song
- Received August 31, 2023 Accepted January 8, 2024 Published online March 4, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1815 [Epub ahead of print]
- 632 View
- 34 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Current research has not investigated the effect of thyroid-stimulating hormone suppression therapy with levothyroxine on the risk for developing subsequent primary cancers (SPCs). This study aimed to investigate the association between levothyroxine dosage and the risk for SPCs in thyroid cancer patients.
Methods
We conducted a nationwide population-based retrospective cohort study form Korean National Health Insurance database. This cohort included 342,920 thyroid cancer patients between 2004 and 2018. Patients were divided into the non-levothyroxine and the levothyroxine groups, the latter consisting of four dosage subgroups according to quartiles. Cox proportional hazard models were performed to evaluate the risk for SPCs by adjusting for variables including cumulative doses of radioactive iodine (RAI) therapy.
Results
A total of 17,410 SPC cases were observed over a median 7.3 years of follow-up. The high-dose levothyroxine subgroups (Q3 and Q4) had a higher risk for SPC (adjusted hazard ratio [HR], 1.14 and 1.27; 95% confidence interval [CI], 1.05–1.24 and 1.17– 1.37; respectively) compared to the non-levothyroxine group. In particular, the adjusted HR of stomach (1.31), colorectal (1.60), liver and biliary tract (1.95), and pancreatic (2.48) cancers were increased in the Q4 subgroup. We consistently observed a positive association between high levothyroxine dosage per body weight and risk of SPCs, even after adjusting for various confounding variables. Moreover, similar results were identified in the stratified analyses according to thyroidectomy type and RAI therapy, as well as in a subgroup analysis of patients with good adherence.
Conclusion
High-dose levothyroxine use was associated with increased risk of SPCs among thyroid cancer patients regardless of RAI therapy.

- Thyroid
- Thyroid Hormone Reference Intervals among Healthy Individuals In Lanzhou, China
- Yan Lu, Wen-Xia Zhang, De-Hong Li, Lian-Hua Wei, Yu-Jun Zhang, Fu-Na Shi, Shen Zhou
- Endocrinol Metab. 2023;38(3):347-356. Published online June 14, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1638
- 1,978 View
- 118 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The common reference intervals (RIs) for thyroid hormones currently used in China are provided by equipment manufacturers. This study aimed to establish thyroid hormone RIs in the population of Lanzhou, a city in the subplateau region of northwest China, and compare them with previous reports and manufacturer-provided values.
Methods
In total, 3,123 individuals (1,680 men, 1,443 women) from Lanzhou, an iodine-adequate area of China, perceived as healthy were selected. The Abbott Architect analyzer was used to determine the serum concentration of thyroid hormones. The 95% RI was estimated using the 2.5th and 97.5th percentiles as the lower and upper reference limits, respectively.
Results
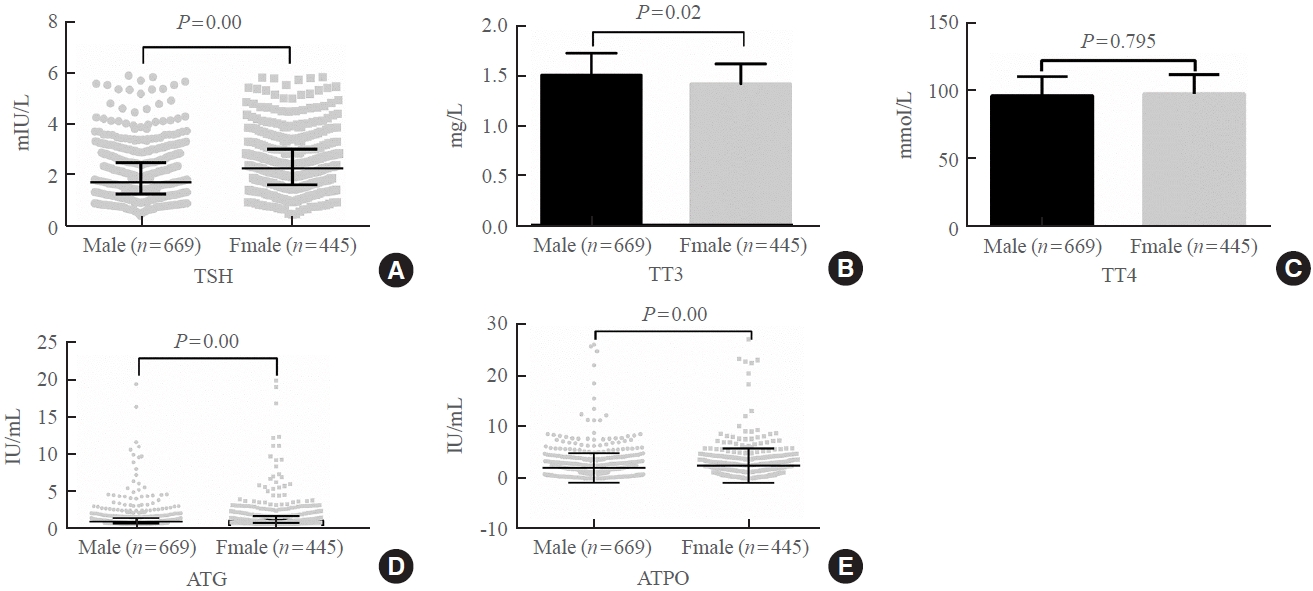
The serum levels of thyroid-stimulating hormone (TSH), total triiodothyronine (TT3), antithyroglobulin (ATG) antibody, and antithyroid peroxidase (ATPO) antibody levels were significantly correlated with sex (P<0.05). TSH, total thyroxine (TT4), and ATPO levels were significantly correlated with age (P<0.05). The serum levels of TSH, ATG, and ATPO in men were significantly lower than in women; in contrast, the serum TT3 level was significantly higher in men than in women (P<0.05). Serum TSH, TT3, TT4, and ATG levels differed across age groups (P<0.05), but no such variation was observed for ATG levels (P>0.05). The established RIs of TSH, ATG, and ATPO in this study differed between sexes (P<0.05). The thyroid hormone RIs established herein were inconsistent with the manufacturer-provided values.
Conclusion
The RIs of thyroid hormones in the healthy population of Lanzhou were inconsistent with those in the manufacturer’s manual. Validated sex-specific values are required for diagnosing thyroid diseases.

- Thyroid
- Evaluation and Management of Bone Health in Patients with Thyroid Diseases: A Position Statement of the Korean Thyroid Association
- A Ram Hong, Ho-Cheol Kang
- Endocrinol Metab. 2023;38(2):175-189. Published online April 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1701
- 3,882 View
- 245 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
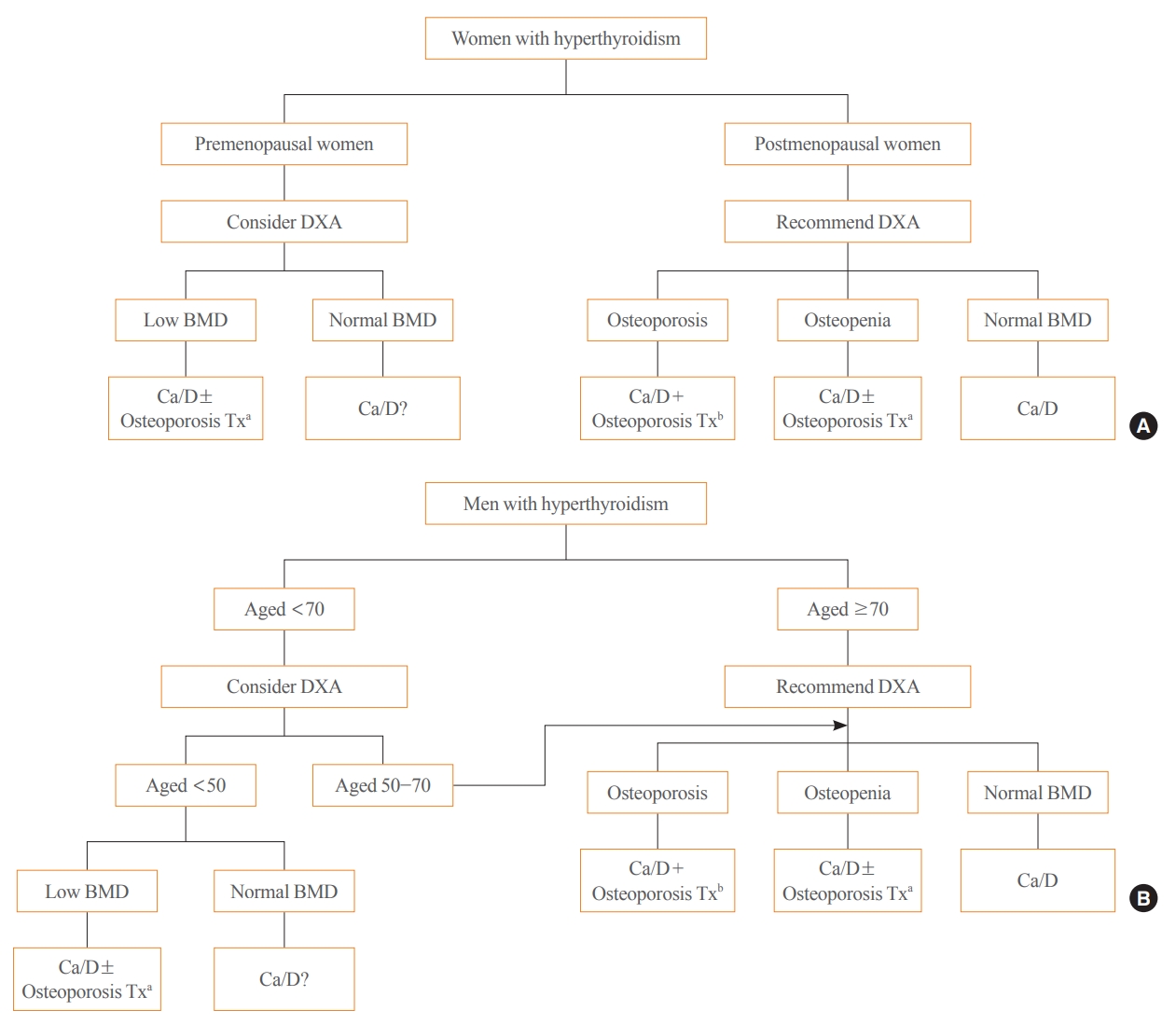
ePub - Thyroid hormones play an important physiological role in maintaining adult bone structure and strength. Consequently, thyroid dysfunction is related to skeletal outcomes. Overt hyperthyroidism is an established cause of high bone turnover with accelerated bone loss, leading to osteoporosis and increased fracture risk. Hyperthyroidism induced by thyroid-stimulating hormone-suppressive therapy in patients with differentiated thyroid cancer is a cause of secondary osteoporosis. In contrast, there is a lack of evidence on the negative impact of hypothyroidism on bone health. Considering the clinical updates on the importance of bone health in thyroid dysfunction, the Task Force from the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association recently developed a position statement on the evaluation and management of bone health of patients with thyroid diseases, particularly focused on endogenous hyperthyroidism and thyroid-stimulating hormone-suppressive therapy-associated hyperthyroidism in patients with differentiated thyroid cancer. Herein, we review the Korean Thyroid Association’s position statement on the evaluation and management of bone health associated with thyroid diseases.
-
Citations
Citations to this article as recorded by- Diagnosis and therapeutic approach to bone health in patients with hypopituitarism
Justyna Kuliczkowska-Płaksej, Aleksandra Zdrojowy-Wełna, Aleksandra Jawiarczyk-Przybyłowska, Łukasz Gojny, Marek Bolanowski
Reviews in Endocrine and Metabolic Disorders.2024;[Epub] CrossRef - Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights
Shunichi Yokota, Hotaka Ishizu, Takuji Miyazaki, Daisuke Takahashi, Norimasa Iwasaki, Tomohiro Shimizu
Biomedicines.2024; 12(4): 843. CrossRef - Review on the protective activity of osthole against the pathogenesis of osteoporosis
Jincai Chen, Xiaofei Liao, Juwen Gan
Frontiers in Pharmacology.2023;[Epub] CrossRef
- Diagnosis and therapeutic approach to bone health in patients with hypopituitarism

- Thyroid
- Thyroid Function across the Lifespan: Do Age-Related Changes Matter?
- John P. Walsh
- Endocrinol Metab. 2022;37(2):208-219. Published online April 14, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1463
- 5,968 View
- 336 Download
- 12 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
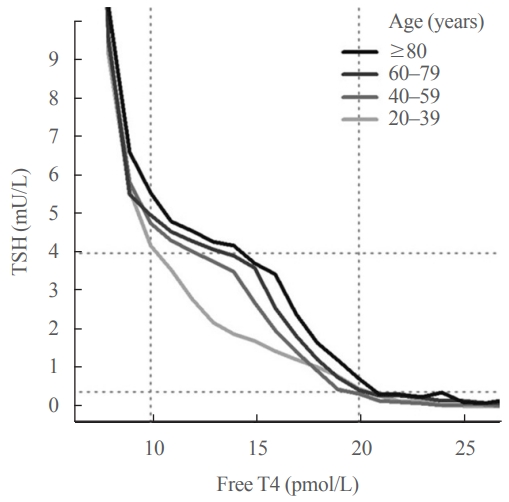
ePub - Circulating concentrations of thyrotropin (TSH) and thyroxine (T4) are tightly regulated. Each individual has setpoints for TSH and free T4 which are genetically determined, and subject to environmental and epigenetic influence. Pituitary-thyroid axis setpoints are probably established in utero, with maturation of thyroid function continuing until late gestation. From neonatal life (characterized by a surge of TSH and T4 secretion) through childhood and adolescence (when free triiodothyronine levels are higher than in adults), thyroid function tests display complex, dynamic patterns which are sexually dimorphic. In later life, TSH increases with age in healthy older adults without an accompanying fall in free T4, indicating alteration in TSH setpoint. In view of this, and evidence that mild subclinical hypothyroidism in older people has no health impact, a strong case can be made for implementation of age-related TSH reference ranges in adults, as is routine in children.
-
Citations
Citations to this article as recorded by- The ageing thyroid: implications for longevity and patient care
Diana van Heemst
Nature Reviews Endocrinology.2024; 20(1): 5. CrossRef - Incidence and Determinants of Spontaneous Normalization of Subclinical Hypothyroidism in Older Adults
Evie van der Spoel, Nicolien A van Vliet, Rosalinde K E Poortvliet, Robert S Du Puy, Wendy P J den Elzen, Terence J Quinn, David J Stott, Naveed Sattar, Patricia M Kearney, Manuel R Blum, Heba Alwan, Nicolas Rodondi, Tinh-Hai Collet, Rudi G J Westendorp,
The Journal of Clinical Endocrinology & Metabolism.2024; 109(3): e1167. CrossRef - Multi-trait analysis characterizes the genetics of thyroid function and identifies causal associations with clinical implications
Rosalie B. T. M. Sterenborg, Inga Steinbrenner, Yong Li, Melissa N. Bujnis, Tatsuhiko Naito, Eirini Marouli, Tessel E. Galesloot, Oladapo Babajide, Laura Andreasen, Arne Astrup, Bjørn Olav Åsvold, Stefania Bandinelli, Marian Beekman, John P. Beilby, Jette
Nature Communications.2024;[Epub] CrossRef - Evaluation of multiple organophosphate insecticide exposure in relation to altered thyroid hormones in NHANES 2007‐2008 adult population
Massira Ousseni Diawara, Songtao Li, Mingzhi Zhang, Francis Manyori Bigambo, Xu Yang, Xu Wang, Tianyu Dong, Di Wu, Chenghao Yan, Yankai Xia
Ecotoxicology and Environmental Safety.2024; 273: 116139. CrossRef - Thyroid-function reference ranges in the diagnosis of thyroid dysfunction in adults
Salman Razvi
Nature Reviews Endocrinology.2024; 20(5): 253. CrossRef - Association between exposure to chemical mixtures and epigenetic ageing biomarkers: Modifying effects of thyroid hormones and physical activity
Wanying Shi, Jianlong Fang, Huimin Ren, Peijie Sun, Juan Liu, Fuchang Deng, Shuyi Zhang, Qiong Wang, Jiaonan Wang, Shilu Tong, Song Tang, Xiaoming Shi
Journal of Hazardous Materials.2024; 469: 134009. CrossRef - DNA Methylation in Autoimmune Thyroid Disease
Nicole Lafontaine, Scott G Wilson, John P Walsh
The Journal of Clinical Endocrinology & Metabolism.2023; 108(3): 604. CrossRef - A Causality between Thyroid Function and Bone Mineral Density in Childhood: Abnormal Thyrotropin May Be Another Pediatric Predictor of Bone Fragility
Dongjin Lee, Moon Ahn
Metabolites.2023; 13(3): 372. CrossRef - Serum Lipidomic Analysis Reveals Biomarkers and Metabolic Pathways of Thyroid Dysfunction
Hua Dong, Wenjie Zhou, Xingxu Yan, Huan Zhao, Honggang Zhao, Yan Jiao, Guijiang Sun, Yubo Li, Zuncheng Zhang
ACS Omega.2023; 8(11): 10355. CrossRef - Developmental and environmental modulation of fecal thyroid hormone levels in wild Assamese macaques (Macaca assamensis)
Verena Behringer, Michael Heistermann, Suchinda Malaivijitnond, Oliver Schülke, Julia Ostner
American Journal of Primatology.2023;[Epub] CrossRef - Prevalence of Functional Alterations and the Effects of Thyroid
Autoimmunity on the Levels of TSH in an Urban Population of Colombia:
A Population-Based Study
Hernando Vargas-Uricoechea, Valentina Agredo-Delgado, Hernando David Vargas-Sierra, María V. Pinzón-Fernández
Endocrine, Metabolic & Immune Disorders - Drug Targets.2023; 23(6): 857. CrossRef - Genetic determinants of thyroid function in children
Tessa A Mulder, Purdey J Campbell, Peter N Taylor, Robin P Peeters, Scott G Wilson, Marco Medici, Colin Dayan, Vincent V W Jaddoe, John P Walsh, Nicholas G Martin, Henning Tiemeier, Tim I M Korevaar
European Journal of Endocrinology.2023; 189(2): 164. CrossRef - Relationship between Thyroid CT Density, Volume, and Future TSH Elevation: A 5-Year Follow-Up Study
Tomohiro Kikuchi, Shouhei Hanaoka, Takahiro Nakao, Yukihiro Nomura, Takeharu Yoshikawa, Md Ashraful Alam, Harushi Mori, Naoto Hayashi
Life.2023; 13(12): 2303. CrossRef - Thyroid Stimulating Hormone and Thyroid Hormones (Triiodothyronine and Thyroxine): An American Thyroid Association-Commissioned Review of Current Clinical and Laboratory Status
Katleen Van Uytfanghe, Joel Ehrenkranz, David Halsall, Kelly Hoff, Tze Ping Loh, Carole A. Spencer, Josef Köhrle
Thyroid®.2023; 33(9): 1013. CrossRef - Blood hormones and suicidal behaviour: A systematic review and meta-analysis
Xue-Lei Fu, Xia Li, Jia-Mei Ji, Hua Wu, Hong-Lin Chen
Neuroscience & Biobehavioral Reviews.2022; 139: 104725. CrossRef
- The ageing thyroid: implications for longevity and patient care

- Diabetes, Obesity and Metabolism
- Effects of Intermittent Fasting on the Circulating Levels and Circadian Rhythms of Hormones
- Bo Hye Kim, Yena Joo, Min-Seon Kim, Han Kyoung Choe, Qingchun Tong, Obin Kwon
- Endocrinol Metab. 2021;36(4):745-756. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.405
- 24,512 View
- 978 Download
- 29 Web of Science
- 29 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
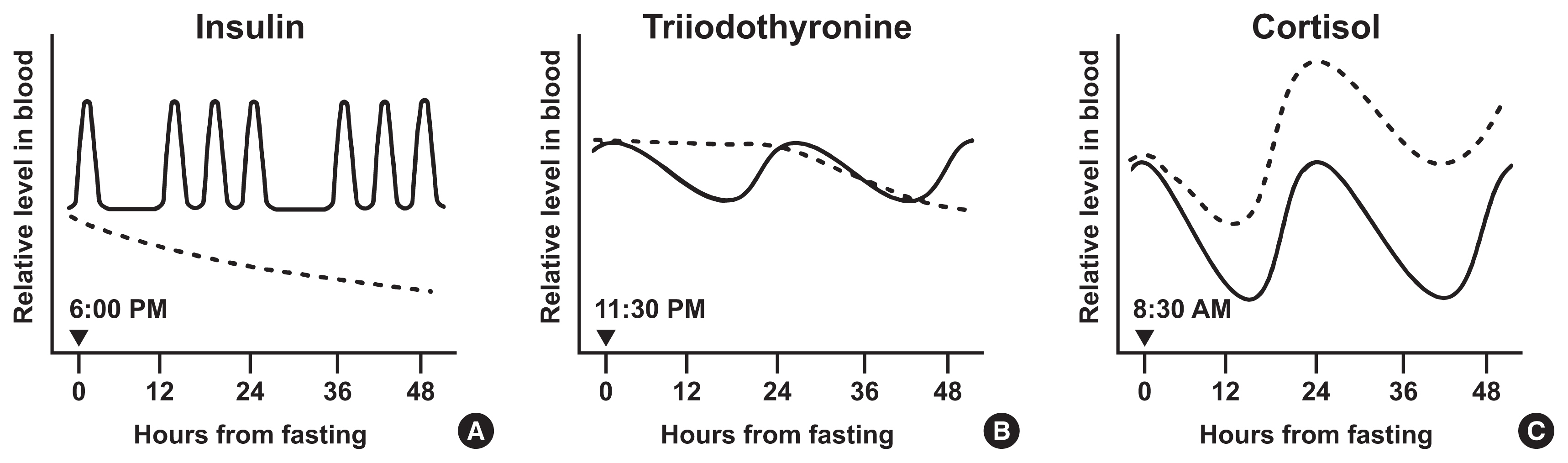
ePub - Intermittent fasting has become an increasingly popular strategy in losing weight and associated reduction in obesity-related medical complications. Overwhelming studies support metabolic improvements from intermittent fasting in blood glucose levels, cardiac and brain function, and other health benefits, in addition to weight loss. However, concerns have also been raised on side effects including muscle loss, ketosis, and electrolyte imbalance. Of particular concern, the effect of intermittent fasting on hormonal circadian rhythms has received little attention. Given the known importance of circadian hormonal changes to normal physiology, potential detrimental effects by dysregulation of hormonal changes deserve careful discussions. In this review, we describe the changes in circadian rhythms of hormones caused by intermittent fasting. We covered major hormones commonly pathophysiologically involved in clinical endocrinology, including insulin, thyroid hormones, and glucocorticoids. Given that intermittent fasting could alter both the level and frequency of hormone secretion, decisions on practicing intermittent fasting should take more considerations on potential detrimental consequences versus beneficial effects pertaining to individual health conditions.
-
Citations
Citations to this article as recorded by- Common and divergent molecular mechanisms of fasting and ketogenic diets
Antonio Paoli, Grant M. Tinsley, Mark P. Mattson, Immaculata De Vivo, Ravi Dhawan, Tatiana Moro
Trends in Endocrinology & Metabolism.2024; 35(2): 125. CrossRef - Identifying Acss1, Mtfp1 and Oxct1 as key regulators and promising biomarkers of sarcopenia in various models
Hailong Cui, Die Hu, Yanling Liu, Jiejie Zhao
Gene.2024; 896: 148053. CrossRef - Circadian Rhythms, Chrononutrition, Physical Training, and Redox Homeostasis—Molecular Mechanisms in Human Health
Cristina Manuela Drăgoi, Alina Crenguţa Nicolae, Anca Ungurianu, Denisa Marilena Margină, Daniela Grădinaru, Ion-Bogdan Dumitrescu
Cells.2024; 13(2): 138. CrossRef - Various types of fasting diet and possible benefits in nonalcoholic fatty liver: Mechanism of actions and literature update
Zahra Sadat Mirrazavi, Vahideh Behrouz
Clinical Nutrition.2024; 43(2): 519. CrossRef - Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
Da Young Lee, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Nan Hee Kim
Diabetes & Metabolism Journal.2024; 48(1): 37. CrossRef - Genetics of Exercise and Diet-Induced Fat Loss Efficiency: A Systematic Review
Aleksandra Bojarczuk, Emiliya S. Egorova, Magdalena Dzitkowska-Zabielska, Ildus I. Ahmetov
Journal of Sports Science and Medicine.2024; : 236. CrossRef - Ramadan fasting in the third trimester of pregnancy and postpartum colostrum cortisol concentrations in Morocco
Meagan M. Guilfoyle
American Journal of Human Biology.2024;[Epub] CrossRef - Dietary factors in circadian rhythm modulation and their impact on metabolic diseases: a state of the science review
Malvika Dalvi, Srujana Medithi
Biological Rhythm Research.2024; : 1. CrossRef - Unlocking the Benefits of Fasting: A Review of its Impact on Various
Biological Systems and Human Health
Rawan Mackieh, Nadia Al-Bakkar, Milena Kfoury, Nathalie Okdeh, Hervé Pietra, Rabih Roufayel, Christian Legros, Ziad Fajloun, Jean-Marc Sabatier
Current Medicinal Chemistry.2024; 31(14): 1781. CrossRef - Fasting intervention and its clinical effects on the human host and microbiome
Sofia K. Forslund
Journal of Internal Medicine.2023; 293(2): 166. CrossRef - Umbrella review of time-restricted eating on weight loss, fasting blood glucose, and lipid profile
Han Shi Jocelyn Chew, Wei How Darryl Ang, Zhen Yang Abel Tan, Wen Wei Ang, Kin Sun Chan, Ying Lau
Nutrition Reviews.2023; 81(9): 1180. CrossRef - Thermodynamic Assessment of the Effects of Intermittent Fasting and Fatty Liver Disease Diets on Longevity
Melek Ece Öngel, Cennet Yildiz, Özge Başer, Bayram Yilmaz, Mustafa Özilgen
Entropy.2023; 25(2): 227. CrossRef - Effects of Intermittent Fasting on Hypothalamus–Pituitary–Thyroid Axis, Palatable Food Intake, and Body Weight in Stressed Rats
Cinthia García-Luna, Ixchel Prieto, Paulina Soberanes-Chávez, Elena Alvarez-Salas, Iván Torre-Villalvazo, Gilberto Matamoros-Trejo, Patricia de Gortari
Nutrients.2023; 15(5): 1164. CrossRef - Possible homeostatic, glucose uptake mechanisms and hepato-pancreatic histological effects of intermittent fasting, exercise, starvation, and honey in streptozotocin-induced diabetes in rats
Ejime A. Chijiokwu, Eze K. Nwangwa, Mega O. Oyovwi, Benneth Ben-Azu, Alexander O. Naiho, Emuesiri Goodies Moke, Victor Emojevwe, Prosper A. Ehiwarior, Udoka S. Nwabuoku
Nutrire.2023;[Epub] CrossRef - Mid-Point of the Active Phase Is Better to Achieve the Natriuretic Effect of Acute Salt Load in Mice
Momoko Imamura, Hiroyuki Sasaki, Katsuki Hayashi, Shigenobu Shibata
Nutrients.2023; 15(7): 1679. CrossRef - All That Glitters Is Not Gold: The Same Sleep Time, but Different Diabetogenic Outcomes
Bohye Kim, Obin Kwon
Endocrinology and Metabolism.2023; 38(1): 78. CrossRef - The emerging role of circadian rhythms in the development and function of thermogenic fat
Xuemin Peng, Yong Chen
Frontiers in Endocrinology.2023;[Epub] CrossRef - Time-restricted Feeding Changes as Inspiration for Drug Design
Zhangyuting He, Huayu Yang, Yilei Mao
Current Pharmaceutical Design.2023; 29(8): 559. CrossRef - Brain Dopamine–Clock Interactions Regulate Cardiometabolic Physiology: Mechanisms of the Observed Cardioprotective Effects of Circadian-Timed Bromocriptine-QR Therapy in Type 2 Diabetes Subjects
Anthony H. Cincotta
International Journal of Molecular Sciences.2023; 24(17): 13255. CrossRef - Adaptive Circadian Rhythms for Autonomous and Biologically Inspired Robot Behavior
Marcos Maroto-Gómez, María Malfaz, Álvaro Castro-González, Sara Carrasco-Martínez, Miguel Ángel Salichs
Biomimetics.2023; 8(5): 413. CrossRef - Intermittent Fasting on Human Health and Disease
Denisa Marilena Margină, Cristina Manuela Drăgoi
Nutrients.2023; 15(21): 4491. CrossRef - Synthetic augmentation of bilirubin metabolism in human pluripotent stem cell-derived liver organoids
Hasan Al Reza, Zishaan Farooqui, Abid Al Reza, Callen Conroy, Kentaro Iwasawa, Yasuhiro Ogura, Keisuke Okita, Kenji Osafune, Takanori Takebe
Stem Cell Reports.2023; 18(11): 2071. CrossRef - Average phenotype but not plasticity in two metabolic hormones covary in wild female bonobos (Pan paniscus)
Ruth Sonnweber, Gottfried Hohmann, Jeroen M. G. Stevens, Tobias Deschner, Barbara Fruth, Anna-Lena Fiedler, Niina O. Nurmi, Verena Behringer
Frontiers in Ecology and Evolution.2023;[Epub] CrossRef - Intermittent fasting, high-intensity interval training, or a combination of both have beneficial effects in obese mice with nonalcoholic fatty liver disease
Patrícia de Castro-de-Paiva, Thatiany de Souza Marinho, Carlos Alberto Mandarim-de-Lacerda, Marcia Barbosa Aguila
The Journal of Nutritional Biochemistry.2022; 104: 108997. CrossRef - Optimal Timing of Thyroid Hormone Replacement During Ramadan Fasting: A Randomized Controlled Trial in Patients with Prior Total Thyroidectomy
Khalid M. Al-Qahtani, Ibraheem Ahmed Aldeeri, Amal M. Alshaibi, Norah Salman Alshabib, Rakan M. Barghouthi, Ebtihal Y. Alyusuf, Anwar Ali Jammah
Thyroid.2022; 32(9): 1029. CrossRef - Exploring the Effects of Energy Constraints on Performance, Body Composition, Endocrinological/Hematological Biomarkers, and Immune System among Athletes: An Overview of the Fasting State
Hadi Nobari, Saber Saedmocheshi, Eugenia Murawska-Ciałowicz, Filipe Manuel Clemente, Katsuhiko Suzuki, Ana Filipa Silva
Nutrients.2022; 14(15): 3197. CrossRef - Alternate day fasting and time-restricted feeding may confer similar neuroprotective effects during aging in male rats
Sukanya Bhoumik, Rashmi Kesherwani, Raushan Kumar, Syed Ibrahim Rizvi
Biogerontology.2022; 23(6): 757. CrossRef - Intermittent Fasting—A Healthy Dietary Pattern for Diabetic Nephropathy
Ming Yang, Wei Chen, Liyu He, Di Liu, Li Zhao, Xi Wang
Nutrients.2022; 14(19): 3995. CrossRef - β-hydroxybutyrate as an Anti-Aging Metabolite
Lian Wang, Peijie Chen, Weihua Xiao
Nutrients.2021; 13(10): 3420. CrossRef
- Common and divergent molecular mechanisms of fasting and ketogenic diets

- Thyroid
- Thyroid Hormone Profile and Its Prognostic Impact on the Coronavirus Disease 2019 in Korean Patients
- Jiyeon Ahn, Min Kyung Lee, Jae Hyuk Lee, Seo Young Sohn
- Endocrinol Metab. 2021;36(4):769-777. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1109
- 4,340 View
- 184 Download
- 17 Web of Science
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
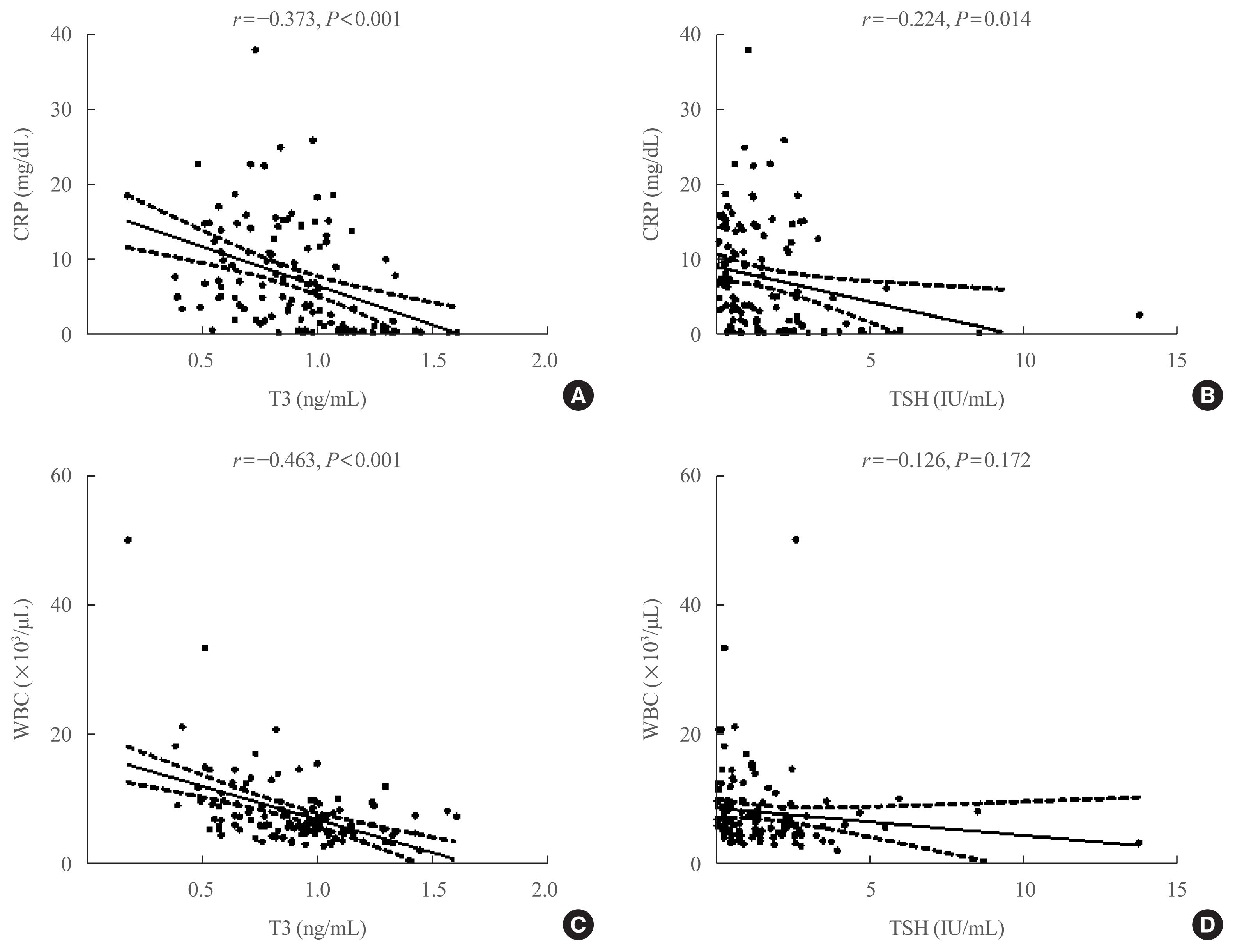
Data on the association between coronavirus disease 2019 (COVID-19) and thyroid have been reported, including overt thyrotoxicosis and suppression of thyroid function. We aimed to evaluate the thyroid hormone profile and its association with the prognosis of COVID-19 in Korean patients.
Methods
The clinical data of 119 patients with COVID-19, admitted in the Myongji Hospital, Goyang, South Korea, were retrospectively evaluated. The thyroid hormone profiles were analyzed and compared based on disease severity (non-severe disease vs. severe to critical disease). Clinical outcomes were analyzed according to the tertiles of thyroid hormones.
Results
Of the 119 patients, 76 (63.9%) were euthyroid, and none presented with overt thyroid dysfunction. Non-thyroidal illness syndrome was the most common manifestation (18.5%), followed by subclinical thyrotoxicosis (14.3%) among patients with thyroid dysfunction. Thyroid stimulating hormone (TSH) and triiodothyronine (T3) levels were significantly lower in patients with severe to critical disease than in those with non-severe disease (P<0.05). Patients in the lowest T3 tertile (<0.77 ng/mL) had higher rates of mechanical ventilation, intensive care unit admission, and death than those in the middle and highest (>1.00 ng/mL) T3 tertiles (P<0.05). COVID-19 patients in the lowest T3 tertile were independently associated with mortality (hazard ratio, 5.27; 95% confidence interval, 1.09 to 25.32; P=0.038) compared with those in the highest T3 tertile.
Conclusion
Thyroid dysfunction is common in COVID-19 patients. Changes in serum TSH and T3 levels may be important markers of disease severity in COVID-19. Decreased T3 levels may have a prognostic significance in COVID-19 related outcome. -
Citations
Citations to this article as recorded by- The prevalence of thyroid disorders in COVID-19 patients: a systematic review and meta-analysis
Sadra Ashrafi, Hossein Hatami, Razieh Bidhendi-Yarandi, Mohammad Hossein Panahi
BMC Endocrine Disorders.2024;[Epub] CrossRef - Thyroid Stimulating Hormone as a Possible Additional COVID-19 Outcome Marker
Anamarija Zrilic Vrkljan, Ana Majic Tengg, Tanja Palaversa, Srecko Marusic, Lana Ruzic, Ines Bilic-Curcic, Maja Cigrovski Berkovic
Medicina.2024; 60(2): 314. CrossRef - Effect of Hypothalamic Adrenal Axis and Thyroid Function Alterations on Prognosis of Critically Ill COVID-19 Patients
Muhammet KORKUSUZ, Sulbiye KARABURGU, Tayfun ET, Rafet YARIMOĞLU, Nuh KUMRU
Namık Kemal Tıp Dergisi.2024; 12(1): 17. CrossRef - Thyroxine changes in COVID-19 pandemic: A systematic review and meta-analysis
Ziqi Li, Pengwei Hou, Shuwen Mu, Renzhi Wang, Hui Miao, Ming Feng, He Wang, Wentai Zhang, Yihao Chen, Tianshun Feng, Shousen Wang, Yi Fang
Frontiers in Endocrinology.2023;[Epub] CrossRef - The Influence of SARS-CoV-2 Infection on the Thyroid Gland
Aleksandra Piekarska, Marta Góral, Marta Kozula, Aleksandra Jawiarczyk-Przybyłowska, Katarzyna Zawadzka, Marek Bolanowski
Biomedicines.2023; 11(2): 614. CrossRef - Thyroid Function Abnormalities and Outcomes in Hospitalized Patients
with COVID-19 Infection: A Cross-Sectional Study
Deepika Patel, Dukhabandhu Naik, Sadishkumar Kamalanathan, Kadhiravan Tamilarasu, Jayaprakash Sahoo, Ayan Roy, Chandhana Merugu, Varun Suryadevara
Hormone and Metabolic Research.2023; 55(03): 169. CrossRef - The Spectrum of Thyroid Function Tests and Autoantibodies During Hospitalization and After Six Months of Discharge in COVID-19 Patients: Does COVID-19 Trigger Autoimmunity?
Ziynet Alphan Uc, Pinar Yagcı, Zelal Adibelli, Cevdet Duran
Endocrine Research.2023; 48(2-3): 44. CrossRef - Transient low T3 syndrome in patients with COVID-19: a new window for prediction of disease severity
Mingyao Zhong, Yue Gao, Hongling Hu, Xuan Zhu, Lulu Gan, Ling Li, Cheng Xiang, Yimin Yan, Zhe Dai
Frontiers in Endocrinology.2023;[Epub] CrossRef - The Association Between COVID-19 and Thyroxine Levels: A Meta-Analysis
Yiru Chen, Xiuneng Li, Yu Dai, Jingjing Zhang
Frontiers in Endocrinology.2022;[Epub] CrossRef - The New Entity of Subacute Thyroiditis amid the COVID-19 Pandemic: From Infection to Vaccine
Mihaela Popescu, Adina Ghemigian, Corina Maria Vasile, Andrei Costache, Mara Carsote, Alice Elena Ghenea
Diagnostics.2022; 12(4): 960. CrossRef - Potential of Endogenous Oxytocin in Endocrine Treatment and Prevention of COVID-19
Stephani C. Wang, Fengmin Zhang, Hui Zhu, Haipeng Yang, Yang Liu, Ping Wang, Vladimir Parpura, Yu-Feng Wang
Frontiers in Endocrinology.2022;[Epub] CrossRef - The Association Between FT3 With the Outcome and Inflammation/Coagulopathy/Fibrinolysis of COVID-19
Jiayi Deng, Siye Zhang, Fei Peng, Quan Zhang, Yi Li, Yanjun Zhong
Frontiers in Endocrinology.2022;[Epub] CrossRef - Primary hypothyroidism with an episode of ventricular tachycardia in a patient with COVID-19
Pin-Hsu Liao, Yu-Cheng Cheng, Po-Yu Liu, I-Te Lee
Medicine.2022; 101(25): e29243. CrossRef - Low triiodothyronine syndrome is associated with stroke‐associated pneumonia
Huijun Chen, Minjie Xu, Yezhi Huang, Jincai He, Wenwei Ren
European Journal of Clinical Investigation.2022;[Epub] CrossRef - Association of thyroid dysfunction and COVID-19: A systematic review and meta-analysis
Mohammad Darvishi, Mohammad Reza Nazer, Hamze Shahali, Majid Nouri
Frontiers in Endocrinology.2022;[Epub] CrossRef - The prognostic utility of serum thyrotropin in hospitalized Covid-19 patients: statistical and machine learning approaches
E. Pappa, P. Gourna, G. Galatas, M. Manti, A. Romiou, L. Panagiotou, R. Chatzikyriakou, N. Trakas, G. Feretzakis, C. Christopoulos
Endocrine.2022; 80(1): 86. CrossRef - Thyrotropin Levels in Patients with Coronavirus Disease 2019: Assessment during Hospitalization and in the Medium Term after Discharge
Abdallah Al-Salameh, Noémie Scherman, Imane Adda, Juliette André, Yoann Zerbib, Julien Maizel, Jean-Daniel Lalau, Etienne Brochot, Claire Andrejak, Rachel Desailloud
Life.2022; 12(12): 2014. CrossRef - COVID-19 and thyroid function: What do we know so far?
Camila Lüdke Rossetti, Juliana Cazarin, Fabio Hecht, Fabyan Esberard de Lima Beltrão, Andrea Cláudia Freitas Ferreira, Rodrigo Soares Fortunato, Helton Estrela Ramos, Denise Pires de Carvalho
Frontiers in Endocrinology.2022;[Epub] CrossRef
- The prevalence of thyroid disorders in COVID-19 patients: a systematic review and meta-analysis

- Thyroid
- The Role of Thyroid Hormone in the Regulation of Cerebellar Development
- Sumiyasu Ishii, Izuki Amano, Noriyuki Koibuchi
- Endocrinol Metab. 2021;36(4):703-716. Published online August 9, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1150
- 4,486 View
- 168 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
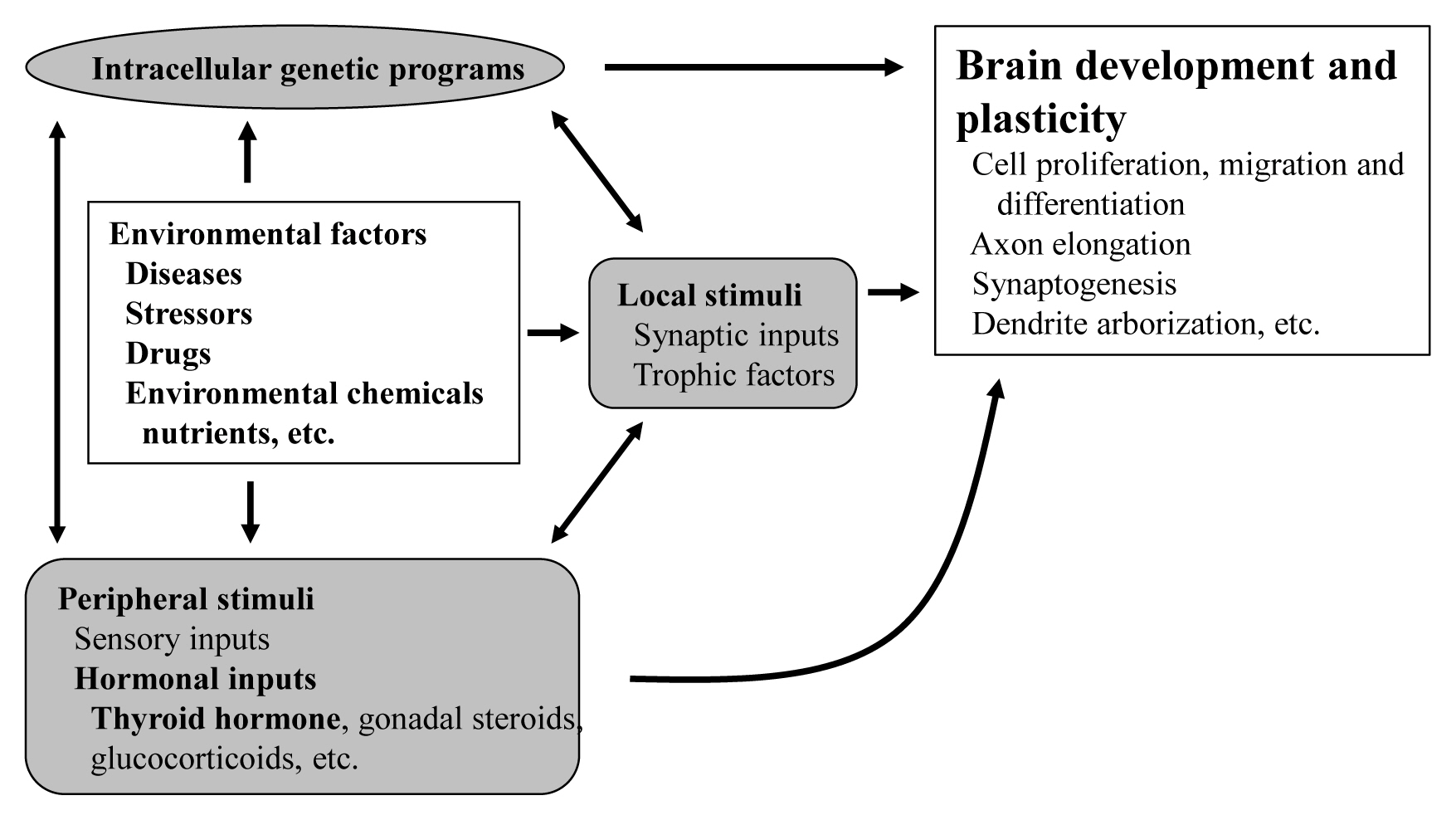
ePub - The proper organized expression of specific genes in time and space is responsible for the organogenesis of the central nervous system including the cerebellum. The epigenetic regulation of gene expression is tightly regulated by an intrinsic intracellular genetic program, local stimuli such as synaptic inputs and trophic factors, and peripheral stimuli from outside of the brain including hormones. Some hormone receptors are expressed in the cerebellum. Thyroid hormones (THs), among numerous circulating hormones, are well-known major regulators of cerebellar development. In both rodents and human, hypothyroidism during the postnatal developmental period results in abnormal morphogenesis or altered function. THs bind to the thyroid hormone receptors (TRs) in the nuclei and with the help of transcriptional cofactors regulate the transcription of target genes. Gene regulation by TR induces cell proliferation, migration, and differentiation, which are necessary for brain development and plasticity. Thus, the lack of TH action mediators may directly cause aberrant cerebellar development. Various kinds of animal models have been established in a bid to study the mechanism of TH action in the cerebellum. Interestingly, the phenotypes differ greatly depending on the models. Herein we summarize the actions of TH and TR particularly in the developing cerebellum.
-
Citations
Citations to this article as recorded by- Neuropeptides and Their Roles in the Cerebellum
Zi-Hao Li, Bin Li, Xiao-Yang Zhang, Jing-Ning Zhu
International Journal of Molecular Sciences.2024; 25(4): 2332. CrossRef - Exploring the underlying molecular mechanism of tri(1,3-dichloropropyl) phosphate-induced neurodevelopmental toxicity via thyroid hormone disruption in zebrafish by multi-omics analysis
Ying Xu, Lei Yang, Yanguo Teng, Jian Li, Na Li
Aquatic Toxicology.2023; 258: 106510. CrossRef - Association of Maternal TSH, FT4 With Children's BMI Trajectories, and Obesity: A Birth Cohort Study
Mengting Yang, Shanshan Zhang, Yuzhu Teng, Xue Ru, Linlin Zhu, Yan Han, Xingyong Tao, Hui Cao, Shuangqin Yan, Fangbiao Tao, Kun Huang
The Journal of Clinical Endocrinology & Metabolism.2023; 109(1): e190. CrossRef - Thyroid hormone receptor beta: Relevance in human health and diseases
Ghausiya Rehman, Neha Kumari, Farhad Bano, Rakesh K. Tyagi
Endocrine and Metabolic Science.2023; 13: 100144. CrossRef - Targeting Thyroid Hormone/Thyroid Hormone Receptor Axis: An Attractive Therapy Strategy in Liver Diseases
Qianyu Tang, Min Zeng, Linxi Chen, Nian Fu
Frontiers in Pharmacology.2022;[Epub] CrossRef - Histone Deacetylase 3 Inhibitor Alleviates Cerebellar Defects in Perinatal Hypothyroid Mice by Stimulating Histone Acetylation and Transcription at Thyroid Hormone-Responsive Gene Loci
Alvin Susetyo, Sumiyasu Ishii, Yuki Fujiwara, Izuki Amano, Noriyuki Koibuchi
International Journal of Molecular Sciences.2022; 23(14): 7869. CrossRef - Selection-driven adaptation to the extreme Antarctic environment in the Emperor penguin
Federica Pirri, Lino Ometto, Silvia Fuselli, Flávia A. N. Fernandes, Lorena Ancona, Nunzio Perta, Daniele Di Marino, Céline Le Bohec, Lorenzo Zane, Emiliano Trucchi
Heredity.2022; 129(6): 317. CrossRef - Long-term depression–inductive stimulation causes long-term potentiation in mouse Purkinje cells with a mutant thyroid hormone receptor
Ayane Ninomiya, Izuki Amano, Michifumi Kokubo, Yusuke Takatsuru, Sumiyasu Ishii, Hirokazu Hirai, Nobutake Hosoi, Noriyuki Koibuchi
Proceedings of the National Academy of Sciences.2022;[Epub] CrossRef
- Neuropeptides and Their Roles in the Cerebellum

- Thyroid
- Bisphenols and Thyroid Hormone
- Min Joo Kim, Young Joo Park
- Endocrinol Metab. 2019;34(4):340-348. Published online December 23, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.4.340
- 9,068 View
- 235 Download
- 60 Web of Science
- 64 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub In recent decades, attention has been directed toward the effects of bisphenol A (BPA) on human health. BPA has estrogenic activity and is regarded as a representative endocrine disruptor. In addition, mounting evidence indicates that BPA can disrupt thyroid hormone and its action. This review examined human epidemiological studies to investigate the association between BPA exposure and thyroid hormone levels, and analyzed
in vivo andin vitro experiments to identify the causal relationship and its mechanism of action. BPA is involved in thyroid hormone action not only as a thyroid hormone receptor antagonist, but also through several other mechanisms. Since the use of bisphenols other than BPA has recently increased, we also reviewed the effects of other bisphenols on thyroid hormone action.-
Citations
Citations to this article as recorded by- The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies
Maria Dalamaga, Dimitrios Kounatidis, Dimitrios Tsilingiris, Natalia G. Vallianou, Irene Karampela, Sotiria Psallida, Athanasios G. Papavassiliou
International Journal of Molecular Sciences.2024; 25(1): 675. CrossRef - Environmental toxicology of bisphenol A: Mechanistic insights and clinical implications on the neuroendocrine system
Tongbing Qi, Dongqing Jing, Kexin Zhang, Junfeng Shi, Hongyan Qiu, Chengxia Kan, Fang Han, Chunyan Wu, Xiaodong Sun
Behavioural Brain Research.2024; 460: 114840. CrossRef - Antioxidant compounds from the Arthrospira platensis protect against Bisphenol A-induced nephrotoxicity in rats
Khadidja Chouala, Kahina Boudjema, Yahia Khelef, Sadok Nani, Kheireddine Ouali, Mahieddine Boumendjel, Amel Boumendjel, Mahfoud Messarah
Toxicology and Environmental Health Sciences.2024; 16(1): 75. CrossRef - Associations of urinary non-persistent endocrine disrupting chemical biomarkers with early-to-mid pregnancy plasma sex-steroid and thyroid hormones
Brad A. Ryva, Diana C. Pacyga, Kaitlyn Y. Anderson, Antonia M. Calafat, Jason Whalen, Max T. Aung, Joseph C. Gardiner, Joseph M. Braun, Susan L. Schantz, Rita S. Strakovsky
Environment International.2024; 183: 108433. CrossRef - Prospective role of lusianthridin in attenuating cadmium-induced functional and cellular damage in rat thyroid
Teng Gao, Sijia Luo, Hongguang Li, Zijie Su, Qinghui Wen
Heliyon.2024; 10(5): e27080. CrossRef - Exposure to Bisphenol A, S, and F and its Association with Obesity and Diabetes Mellitus in General Adults of Korea: Korean National Environmental Health Survey (KoNEHS) 2015–2017
Min Kyong Moon, Min Joo Kim, Inae Lee, Sunmi Kim, Sohyeon Choi, Jeongim Park, Yoon Hee Cho, Sooyeon Hong, Jiyoung Yoo, Hyunwoong Park, Gi Jeong Cheon, Young Joo Park, Kyungho Choi
Exposure and Health.2023; 15(1): 53. CrossRef - Exposure to Bisphenol A increases malignancy risk of thyroid nodules in overweight/obese patients
Vincenzo Marotta, Lucia Grumetto, Ilaria Neri, Giacomo Russo, Anna Tortora, Giulia Izzo, Ilaria Panariello, Domenico Rocco, Luciano Pezzullo, Mario Vitale
Environmental Pollution.2023; 316: 120478. CrossRef - A case-control study of urinary concentrations of bisphenol A, bisphenol F, and bisphenol S and the risk of papillary thyroid cancer
Lei Zhang, Jiahuai Zhang, Sai Fan, Yuxin Zhong, Jingguang Li, Yunfeng Zhao, Song Ni, Jiaying Liu, Yongning Wu
Chemosphere.2023; 312: 137162. CrossRef - Endocrine disruptors and endometriosis
Sudipta Dutta, Sakhila K. Banu, Joe A. Arosh
Reproductive Toxicology.2023; 115: 56. CrossRef - Bisphenol A alternatives continuously contribute to the endocrine disruption in cetaceans
Yongwei Guo, Wei Shi, Zhiwei Liu, Xian Sun, Jiaxue Wu, Yuping Wu
Environment International.2023; 171: 107679. CrossRef - Environmental endocrine disruptors and amphibian immunity: A bridge between the thyroid hormone axis and T cell development
Connor C. McGuire, Jacques R. Robert
Developmental & Comparative Immunology.2023; 140: 104617. CrossRef - Transient developmental exposure to low doses of bisphenol F negatively affects neurogliogenesis and olfactory behaviour in adult mice
Pieter Vancamp, Lucile Butruille, Anni Herranen, Anita Boelen, Jean-Baptiste Fini, Barbara A. Demeneix, Sylvie Remaud
Environment International.2023; 172: 107770. CrossRef - A Fast Method for Determination of Seven Bisphenols in Human Breast Milk Samples with the Use of HPLC-FLD
Szymon Szubartowski, Tomasz Tuzimski
Molecules.2023; 28(3): 1432. CrossRef - Risk Assessment of Bisphenol A in the Korean General Population
Myungsil Hwang, Seon-Joo Park, Hae-Jeung Lee
Applied Sciences.2023; 13(6): 3587. CrossRef - Current Evidence on Bisphenol A Exposure and the Molecular Mechanism Involved in Related Pathological Conditions
Ylenia Della Rocca, Enrico Matteo Traini, Francesca Diomede, Luigia Fonticoli, Oriana Trubiani, Alessia Paganelli, Jacopo Pizzicannella, Guya Diletta Marconi
Pharmaceutics.2023; 15(3): 908. CrossRef - The associations between concentrations of gestational bisphenol analogues and thyroid related hormones in cord blood: A prospective cohort study
Jianya Xi, Xiujuan Su, Ziliang Wang, Honglei Ji, Yao Chen, Xiaofang Liu, Maohua Miao, Hong Liang, Wei Yuan
Ecotoxicology and Environmental Safety.2023; 256: 114838. CrossRef - Zebrafish (Danio rerio) TRβ- and TTR-based electrochemical biosensors: Construction and application for the evaluation of thyroid-disrupting activity of bisphenols
Yuejiao Li, Zhenzhong Zhang, Jun Wang, Yeqi Shan, Hua Tian, Pengfei Cui, Shaoguo Ru
Environmental Pollution.2023; 330: 121745. CrossRef - Iodine Deficiency, Maternal Hypothyroxinemia and Endocrine Disrupters Affecting Fetal Brain Development: A Scoping Review
Rolf Grossklaus, Klaus-Peter Liesenkötter, Klaus Doubek, Henry Völzke, Roland Gaertner
Nutrients.2023; 15(10): 2249. CrossRef - Bisphenol A Analogues Inhibit Human and Rat 11β-Hydroxysteroid Dehydrogenase 1 Depending on Its Lipophilicity
Hong Wang, Jianmin Sang, Zhongyao Ji, Yang Yu, Shaowei Wang, Yang Zhu, Huitao Li, Yiyan Wang, Qiqi Zhu, Renshan Ge
Molecules.2023; 28(13): 4894. CrossRef - Bisphenol A-Induced Endocrine Dysfunction and its Associated Metabolic Disorders
Meenu Maniradhan, Latchoumycandane Calivarathan
Endocrine, Metabolic & Immune Disorders - Drug Targets.2023; 23(4): 515. CrossRef - Origin, dietary exposure, and toxicity of endocrine-disrupting food chemical contaminants: A comprehensive review
Leila Peivasteh-roudsari, Raziyeh Barzegar-bafrouei, Kurush Aghbolagh Sharifi, Shamimeh Azimisalim, Marziyeh Karami, Solmaz Abedinzadeh, Shabnam Asadinezhad, Behrouz Tajdar-oranj, Vahideh Mahdavi, Adel Mirza Alizadeh, Parisa Sadighara, Margherita Ferrante
Heliyon.2023; 9(7): e18140. CrossRef - Bisphenol analogues induce thyroid dysfunction via the disruption of the thyroid hormone synthesis pathway
Chao Hu, Yeqing Xu, Mingmin Wang, Shixuan Cui, Hangjun Zhang, Liping Lu
Science of The Total Environment.2023; 900: 165711. CrossRef - Distinct inhibitory strength of bisphenol A analogues on human and rat 11β-hydroxysteroid dehydrogenase 1: 3D quantitative structure-activity relationship and in silico molecular docking analysis
Han Lu, Shaowei Wang, Jingyi Zheng, Yang Zhu, Yiyan Wang, Huitao Li, Ren-shan Ge
Ecotoxicology and Environmental Safety.2023; 267: 115638. CrossRef - Systematic Review on Safety of Bisphenol A: from Invention to the Present
Hananeh KORDBACHEH, Bensu KARAHALİL
Eurasian Journal of Toxicology.2023; 5(2): 37. CrossRef - Bisphenol S dysregulates thyroid hormone homeostasis; Testicular survival, redox and metabolic status: Ameliorative actions of melatonin
Aishwarya Sahu, Rakesh Verma
Environmental Toxicology and Pharmacology.2023; 104: 104300. CrossRef - Structural binding perspectives of common plasticizers and a flame retardant, BDE‐153, against thyroxine‐binding globulin: potential for endocrine disruption
Ishfaq Ahmad Sheikh, Mohd Amin Beg
Journal of Applied Toxicology.2022; 42(5): 841. CrossRef - New insights on the effects of endocrine-disrupting chemicals on children
Barbara Predieri, Crésio A.D. Alves, Lorenzo Iughetti
Jornal de Pediatria.2022; 98: S73. CrossRef - Toxic Metal Concentrations in Drinking Water and Possible Effect on Sex Hormones among Men in Sabongida-Ora, Edo State, Nigeria
Osaro Ogie Enehizena, Mathias A. Emokpae
Medicines.2022; 9(1): 4. CrossRef - Bisphenols impact hormone levels in animals: A meta-analysis
Alexander M. Rubin, Frank Seebacher
Science of The Total Environment.2022; 828: 154533. CrossRef - The effects of prenatal and lactational bisphenol A and/or di(2-ethylhexyl) phthalate exposure on female reproductive system
Gizem Ozkemahli, Aylin Balci Ozyurt, Pinar Erkekoglu, Naciye Dilara Zeybek, Nilgun Yersal, Belma Kocer-Gumusel
Toxicology Mechanisms and Methods.2022; 32(8): 597. CrossRef - Associations of Phthalate Metabolites and Bisphenol A Levels with Obesity in Children: The Korean National Environmental Health Survey (KoNEHS) 2015 to 2017
Moon Young Seo, Shinje Moon, Shin-Hye Kim, Mi Jung Park
Endocrinology and Metabolism.2022; 37(2): 249. CrossRef - Associations of bisphenol exposure with thyroid hormones in pregnant women: a prospective birth cohort study in China
Huishen Huang, Jun Liang, Peng Tang, Chuanxiang Yu, Haoran Fan, Qian Liao, Jinghua Long, Dongxiang Pan, Xiaoyun Zeng, Shun Liu, Dongping Huang, Xiaoqiang Qiu
Environmental Science and Pollution Research.2022; 29(58): 87170. CrossRef - Bisphenols A, F, S and AF trigger apoptosis and/or endoplasmic reticulum stress in human endometrial stromal cells
Ricardo Ferreira, Cristina Amaral, Georgina Correia-da-Silva, Marta Almada, Margarida Borges, Sara Cristina Cunha, José Oliveira Fernandes, Natércia Teixeira
Toxicology.2022; 478: 153282. CrossRef - Association between phenols and thyroid hormones: The role of iodothyronine deiodinase genes
Blanca Sarzo, Reem Abumallouh, Natalia Marín, Sabrina Llop, Andrea Beneito, Inmaculada Lopez-Flores, Nerea Ferrero, Amrit Kaur Sakhi, Ferran Ballester, Maria-Jose Lopez-Espinosa
Environmental Pollution.2022; 311: 119926. CrossRef - Bisphenol A as a Factor in the Mosaic of Autoimmunity
Zora Lazurova, Ivica Lazurova, Yehuda Shoenfeld
Endocrine, Metabolic & Immune Disorders - Drug Targets.2022; 22(7): 728. CrossRef - Zebrafish as an emerging tool for drug discovery and development for thyroid diseases
Poonam Yadav, Lopmudra P. Sarode, Ravinder Reddy Gaddam, Puneet Kumar, Jasvinder Singh Bhatti, Amit Khurana, Umashanker Navik
Fish & Shellfish Immunology.2022; 130: 53. CrossRef - Review of in silico studies dedicated to the nuclear receptor family: Therapeutic prospects and toxicological concerns
Asma Sellami, Manon Réau, Matthieu Montes, Nathalie Lagarde
Frontiers in Endocrinology.2022;[Epub] CrossRef - Use of high-resolution metabolomics to assess the biological perturbations associated with maternal exposure to Bisphenol A and Bisphenol F among pregnant African American women
Rachel Tchen, Youran Tan, Dana Boyd Barr, P. Barry Ryan, ViLinh Tran, Zhenjiang Li, Yi-Juan Hu, Alicia K. Smith, Dean P. Jones, Anne L. Dunlop, Donghai Liang
Environment International.2022; 169: 107530. CrossRef - Effects of bisphenol A on pancreas and thyroid gland of young and adult female Sprague Dawlеy rats
D. Yahia, H. Hamdy, D. A. Salem, S. Afifi
BULGARIAN JOURNAL OF VETERINARY MEDICINE.2022; 25(3): 426. CrossRef - Bisphenol A analogues induce a feed-forward estrogenic response in zebrafish
Silvia Karim, Ruixin Hao, Caroline Pinto, Jan-Åke Gustafsson, Marina Grimaldi, Patrick Balaguer, Maria Bondesson
Toxicology and Applied Pharmacology.2022; 455: 116263. CrossRef - Mediterranean Diet and Thyroid: An Interesting Alliance
Giuseppe Bellastella, Lorenzo Scappaticcio, Francesco Caiazzo, Maria Tomasuolo, Raffaela Carotenuto, Mariangela Caputo, Stefania Arena, Paola Caruso, Maria Ida Maiorino, Katherine Esposito
Nutrients.2022; 14(19): 4130. CrossRef - Endocrine Disrupting Chemicals’ Effects in Children: What We Know and What We Need to Learn?
Barbara Predieri, Lorenzo Iughetti, Sergio Bernasconi, Maria Elisabeth Street
International Journal of Molecular Sciences.2022; 23(19): 11899. CrossRef - Single and repeated bisphenol A treatment induces ROS, Aβ and hyperphosphorylated-tau accumulation, and insulin pathways disruption, through HDAC2 and PTP1B overexpression, leading to SN56 cholinergic apoptotic cell death
Andrea Flores, Paula Moyano, Emma Sola, José Manuel García, Jimena García, María José Anadon, María Teresa Frejo, Maria Victoria Naval, Maria de la Cabeza Fernadez, Javier del Pino
Food and Chemical Toxicology.2022; 170: 113500. CrossRef - Application of High-Performance Liquid Chromatography Combined with Fluorescence Detector and Dispersive Liquid–Liquid Microextraction to Quantification of Selected Bisphenols in Human Amniotic Fluid Samples
Szymon Szubartowski, Tomasz Tuzimski
International Journal of Environmental Research and Public Health.2022; 20(1): 297. CrossRef - The Association between Phenols and Thyroid Hormones: The Role of Iodothyronine Deiodinase Genes
Blanca Sarzo, Reem Abumallouh, Natalia Marin, Sabrina Llop, Andrea Beneito, Inmaculada Lopez-Flores, Nerea Ferrero, Amrit Kaur Sakhi, ferran ballester, Maria-Jose Lopez-Espinosa
SSRN Electronic Journal .2022;[Epub] CrossRef - Bisphenols emerging in Norwegian and Czech aquatic environments show transthyretin binding potency and other less-studied endocrine-disrupting activities
Pavel Šauer, Helena Švecová, Kateřina Grabicová, Farah Gönül Aydın, Tomáš Mackuľak, Vít Kodeš, Line Diana Blytt, Liv Bruås Henninge, Roman Grabic, Hana Kocour Kroupová
Science of The Total Environment.2021; 751: 141801. CrossRef - Endocrine Disrupting Chemicals and Thyroid Cancer: An Overview
Mathilda Alsen, Catherine Sinclair, Peter Cooke, Kimia Ziadkhanpour, Eric Genden, Maaike van Gerwen
Toxics.2021; 9(1): 14. CrossRef - Thyroid-Modulating Activities of Olive and Its Polyphenols: A Systematic Review
Kok-Lun Pang, Johanna Nathania Lumintang, Kok-Yong Chin
Nutrients.2021; 13(2): 529. CrossRef - Human biomonitoring of bisphenol A along pregnancy: An exposure reconstruction of the EXHES-Spain cohort
María Ángeles Martínez, Neus González, Anna Martí, Montse Marquès, Joaquim Rovira, Vikas Kumar, Martí Nadal
Environmental Research.2021; 196: 110941. CrossRef - Dietary Intake of Endocrine Disrupting Substances Presents in Environment and Their Impact on Thyroid Function
Aneta Sokal, Sara Jarmakiewicz-Czaja, Jacek Tabarkiewicz, Rafał Filip
Nutrients.2021; 13(3): 867. CrossRef - BPA and BPA alternatives BPS, BPAF, and TMBPF, induce cytotoxicity and apoptosis in rat and human stem cells
Kristen G. Harnett, Ashley Chin, Sonya M. Schuh
Ecotoxicology and Environmental Safety.2021; 216: 112210. CrossRef - Bisphenols and the Development of Type 2 Diabetes: The Role of the Skeletal Muscle and Adipose Tissue
Fozia Ahmed, Maria Pereira, Céline Aguer
Environments.2021; 8(4): 35. CrossRef - Involvement of Thyroid Hormones in Brain Development and Cancer
Gabriella Schiera, Carlo Maria Di Liegro, Italia Di Liegro
Cancers.2021; 13(11): 2693. CrossRef - Environmental Factors Affecting Thyroid-Stimulating Hormone and Thyroid Hormone Levels
Mirjana Babić Leko, Ivana Gunjača, Nikolina Pleić, Tatijana Zemunik
International Journal of Molecular Sciences.2021; 22(12): 6521. CrossRef - Thyroid health in big city realities
Liudmila L. Kamynina
City Healthcare.2021; 2(2): 84. CrossRef - Adverse effects of bisphenol B exposure on the thyroid and nervous system in early life stages of zebrafish
Qian Yang, Zhenzhu Zhu, Qin Liu, Lihong Chen
Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology.2021; 250: 109167. CrossRef - Teratogenicity and toxicity of the new BPA alternative TMBPF, and BPA, BPS, and BPAF in chick embryonic development
Kristen G. Harnett, Lucy G. Moore, Ashley Chin, Isabel C. Cohen, Rylee R. Lautrup, Sonya M. Schuh
Current Research in Toxicology.2021; 2: 399. CrossRef - A Review on the Occurrence, Exposure, and Health Impacts of Bisphenol A
Prince Sharma, Khushboo Sharma, Geetika Sharma, Pooja Chadha
Toxicology International.2021; : 337. CrossRef - Thyroxine-binding globulin, peripheral deiodinase activity, and thyroid autoantibody status in association of phthalates and phenolic compounds with thyroid hormones in adult population
Sohyeon Choi, Min Joo Kim, Young Joo Park, Sunmi Kim, Kyungho Choi, Gi Jeong Cheon, Yoon Hee Cho, Hye Li Jeon, Jiyoung Yoo, Jeongim Park
Environment International.2020; 140: 105783. CrossRef - Clinical expression of endocrine disruptors in children
Lorenzo Iughetti, Laura Lucaccioni, Maria E. Street, Sergio Bernasconi
Current Opinion in Pediatrics.2020; 32(4): 554. CrossRef - Combined effects of di (2-ethylhexyl) phthalate and bisphenol A on thyroid hormone homeostasis in adolescent female rats
Xuan Zhang, Yuejiao Zhao, Cheng Cheng, Liuli Li, Mingyang Xiao, Guopei Zhang, Xiaobo Lu
Environmental Science and Pollution Research.2020; 27(32): 40882. CrossRef - How microplastic components influence the immune system and impact on children health: Focus on cancer
Mariana Segovia‐Mendoza, Karen E. Nava‐Castro, Margarita I. Palacios‐Arreola, Claudia Garay‐Canales, Jorge Morales‐Montor
Birth Defects Research.2020; 112(17): 1341. CrossRef - Perinatal exposure to Bisphenol A disturbs the early differentiation of male germ cells
Romina Pagotto, Clarisa G. Santamaría, María Belén Harreguy, Julián Abud, María Laura Zenclussen, Laura Kass, Martina Crispo, Mónica M. Muñoz-de-Toro, Horacio A. Rodriguez, Mariela Bollati-Fogolín
Reproductive Toxicology.2020; 98: 117. CrossRef - Novel Biobased Furanic Diols as Potential Alternatives to BPA: Synthesis and Endocrine Activity Screening
Catherine A. Sutton, Alexander Polykarpov, Keimpe Jan van den Berg, Alexander Yahkind, Linda J. Lea, Dean C. Webster, Mukund P. Sibi
ACS Sustainable Chemistry & Engineering.2020; 8(51): 18824. CrossRef
- The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies

- Thyroid
- Evaluation of Thyroid Hormone Levels and Urinary Iodine Concentrations in Koreans Based on the Data from Korea National Health and Nutrition Examination Survey VI (2013 to 2015)
- Jae Hoon Chung
- Endocrinol Metab. 2018;33(2):160-163. Published online May 4, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.2.160
- 4,041 View
- 49 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub No nationwide data have been published about thyroid hormone levels and urinary iodine concentrations (UICs) in Korea. The Korea Centers for Disease Control and Prevention and the Korean Thyroid Association established a project to evaluate the nationwide thyroid hormone profile and UICs in healthy Koreans as part of the Korea National Health and Nutrition Examination Survey (KNHANES) VI (2013 to 2015), a nationwide, cross-sectional survey of the Korean population that enrolled 7,061 individuals who were weighted to represent the entire Korean population. Based on the KNHANES VI, the geometric mean value of serum thyroid stimulating hormone was 2.16 mIU/L, and its reference interval was 0.59 to 7.03 mIU/L. The mean value of serum free thyroxine was 1.25 ng/dL, and its reference interval was 0.92 to 1.60 ng/dL. The median UIC in the Korean population was reported to be 294 μg/L, corresponding to ‘above requirements’ iodine intake according to the World Health Organization recommendations. A U-shaped relationship of UIC with age was found. The prevalence of overt hyperthyroidism and overt hypothyroidism in the Korean population based on the KNHANES VI was 0.54% and 0.73%, respectively.
-
Citations
Citations to this article as recorded by- Correlation between shift work and non-alcoholic fatty liver disease among male workers in the steel manufacturing company of Korea: a cross-sectional study
Kiseok Kim, Yong-Jin Lee, Soon-Chan Kwon, Young-Sun Min, Hyun Kyo Lee, Gwangin Baek, Sang Hyeon Kim, Eun-Chul Jang
Annals of Occupational and Environmental Medicine.2022;[Epub] CrossRef - Subclinical Hypothyroidism: Prevalence, Health Impact, and Treatment Landscape
Won Sang Yoo, Hyun Kyung Chung
Endocrinology and Metabolism.2021; 36(3): 500. CrossRef - T4+T3 Combination Therapy: An Unsolved Problem of Increasing Magnitude and Complexity
Wilmar M. Wiersinga
Endocrinology and Metabolism.2021; 36(5): 938. CrossRef - Update on Thyroid Hormone Levels and Thyroid Dysfunction in the Korean Population Based on Data from the Korea National Health and Nutrition Examination Survey VI (2013 to 2015)
Jae Hoon Chung
Endocrinology and Metabolism.2020; 35(1): 7. CrossRef - Different Relationships Between Thyrotropin and Muscle Strength According to Sex and Age in Euthyroid Koreans (The 6th Korea National Health and Nutritional Examination Survey 2014–2015)
Seong Hee Ahn, Da Hea Seo, Yongin Cho, Mihye Jung, So Hun Kim, Seongbin Hong
Thyroid.2020; 30(12): 1710. CrossRef
- Correlation between shift work and non-alcoholic fatty liver disease among male workers in the steel manufacturing company of Korea: a cross-sectional study

- Clinical Study
- Triiodothyronine Levels Are Independently Associated with Metabolic Syndrome in Euthyroid Middle-Aged Subjects
- Hye Jeong Kim, Ji Cheol Bae, Hyeong Kyu Park, Dong Won Byun, Kyoil Suh, Myung Hi Yoo, Jae Hyeon Kim, Yong-Ki Min, Sun Wook Kim, Jae Hoon Chung
- Endocrinol Metab. 2016;31(2):311-319. Published online May 13, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.311
- 4,443 View
- 32 Download
- 25 Web of Science
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Recent studies have shown an association between thyroid hormone levels and metabolic syndrome (MetS) among euthyroid individuals; however, there have been some inconsistencies between studies. Here, we evaluated the relationship between thyroid hormone levels and MetS in euthyroid middle-aged subjects in a large cohort.
Methods A retrospective analysis of 13,496 euthyroid middle-aged subjects who participated in comprehensive health examinations was performed. Subjects were grouped according to thyroid stimulating hormone, total triiodothyronine (T3), total thyroxine (T4), and T3-to-T4 ratio quartile categories. We estimated the odds ratios (ORs) for MetS according to thyroid hormone quartiles using logistic regression models, adjusted for potential confounders.
Results Of the study patients, 12% (
n =1,664) had MetS. A higher T3 level and T3-to-T4 ratio were associated with unfavourable metabolic profiles, such as higher body mass index, systolic and diastolic blood pressure, triglycerides, fasting glucose and glycated hemoglobin, and lower high density lipoprotein cholesterol levels. The proportion of participants with MetS increased across the T3 quartile categories (P for trend <0.001) and the T3-to-T4 ratio quartile categories (P for trend <0.001). The multi-variate-adjusted OR (95% confidence interval) for MetS in the highest T3 quartile group was 1.249 (1.020 to 1.529) compared to the lowest T3 quartile group, and that in the highest T3-to-T4 ratio quartile group was 1.458 (1.141 to 1.863) compared to the lowest T3-to-T4 ratio quartile group, even after adjustment for potential confounders.Conclusion Serum T3 levels and T3-to-T4 ratio are independently associated with MetS in euthyroid middle-aged subjects. Longitudinal studies are needed to define this association and its potential health implications.
-
Citations
Citations to this article as recorded by- The effect of endocrine disrupting chemicals on the vitronectin-receptor (integrin αvβ3)-mediated cell adhesion of human umbilical vein endothelial cells
Maša Kenda, Urša Pečar Fonović, Janko Kos, Marija Sollner Dolenc
Toxicology in Vitro.2022; 79: 105275. CrossRef - Could the ketogenic diet induce a shift in thyroid function and support a metabolic advantage in healthy participants? A pilot randomized-controlled-crossover trial
Stella Iacovides, Shane K. Maloney, Sindeep Bhana, Zareena Angamia, Rebecca M. Meiring, Carla Pegoraro
PLOS ONE.2022; 17(6): e0269440. CrossRef - Mediation effects of thyroid function in the associations between phthalate exposure and lipid metabolism in adults
Han-Bin Huang, Po-Keng Cheng, Chi-Ying Siao, Yuan-Ting C. Lo, Wei-Chun Chou, Po-Chin Huang
Environmental Health.2022;[Epub] CrossRef - Cholinesterase homozygous genotype as susceptible biomarker of hypertriglyceridaemia for pesticide-exposed agricultural workers
Xingfan Zhou, Min Zhang, Yuqian Wang, Hailing Xia, Lijin Zhu, Guangyi Li, Li Rong, Huahuang Dong, Rui Chen, Shichuan Tang, Min Yu
Biomarkers.2021; 26(4): 335. CrossRef - Association between thyroid hormone and components of metabolic syndrome in euthyroid Korean adults
Kyung A. Shin, Eun Jae Kim
Medicine.2021; 100(51): e28409. CrossRef - Clinical Parameters Are More Likely to Be Associated with Thyroid Hormone Levels than with Thyrotropin Levels: A Systematic Review and Meta-Analysis
Stephen P. Fitzgerald, Nigel G. Bean, Henrik Falhammar, Jono Tuke
Thyroid.2020; 30(12): 1695. CrossRef - The role of thyroid hormone in metabolism and metabolic syndrome
Patrícia de Fátima dos Santos Teixeira, Patrícia Borges dos Santos, Carmen Cabanelas Pazos-Moura
Therapeutic Advances in Endocrinology and Metabolism.2020; 11: 204201882091786. CrossRef - Association between Abdominal Fat Distribution and Free Triiodothyronine in a Euthyroid Population
Xiaomin Nie, Yiting Xu, Xiaojing Ma, Yunfeng Xiao, Yufei Wang, Yuqian Bao
Obesity Facts.2020; 13(3): 358. CrossRef - Association of thyroid function with white coat hypertension and sustained hypertension
Peng Cai, Yan Peng, YuXi Chen, Li Li, Wei Chu, Yan Wang, Xukai Wang
The Journal of Clinical Hypertension.2019; 21(5): 674. CrossRef - Thyroid function is associated with body mass index and fasting plasma glucose in Thai euthyroid population
Amornpan Lertrit, La-or Chailurkit, Boonsong Ongphiphadhanakul, Wichai Aekplakorn, Chutintorn Sriphrapradang
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(1): 468. CrossRef - Thyroid disease and the metabolic syndrome
Ladan Mehran, Atieh Amouzegar, Fereidoun Azizi
Current Opinion in Endocrinology, Diabetes & Obesity.2019; 26(5): 256. CrossRef - Morbid obez hastalarda kilo kaybının insulin direnci, bazal metabolizma hızı, antropometrik ölçümler ve tiroid fonksiyonlarına etkisi
Şenay DURMAZ CEYLAN, Şuuri Ahsen CEYLAN, Fatih EKER, Aşkın GÜNGÜNEŞ
Anadolu Güncel Tıp Dergisi.2019; 1(4): 99. CrossRef - Body Composition, Resting Energy Expenditure, and Metabolic Changes in Women Diagnosed with Differentiated Thyroid Carcinoma
Elena Izkhakov, Nachum Vaisman, Sophie Barnes, Micha Barchana, Naftali Stern, Lital Keinan-Boker
Thyroid.2019; 29(8): 1044. CrossRef - High TSH Level within Normal Range Is Associated with Obesity, Dyslipidemia, Hypertension, Inflammation, Hypercoagulability, and the Metabolic Syndrome: A Novel Cardiometabolic Marker
Yi-Cheng Chang, Shih-Che Hua, Chia-Hsuin Chang, Wei-Yi Kao, Hsiao-Lin Lee, Lee-Ming Chuang, Yen-Tsung Huang, Mei-Shu Lai
Journal of Clinical Medicine.2019; 8(6): 817. CrossRef - Metabolic Syndrome, Thyroid Function and Autoimmunity - The PORMETS Study
Luís Raposo, Sandra Martins, Daniela Ferreira, João Tiago Guimarães, Ana Cristina Santos
Endocrine, Metabolic & Immune Disorders - Drug Targets.2019; 19(1): 75. CrossRef - Hormesis in Health and Chronic Diseases
Xin Li, Tingting Yang, Zheng Sun
Trends in Endocrinology & Metabolism.2019; 30(12): 944. CrossRef - Relationship of metabolic syndrome and its components with thyroid dysfunction in Algerian patients
Mohamed Larbi Hamlaoui, Ammar Ayachi, Aoulia Dekaken, Adel Gouri
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2018; 12(1): 1. CrossRef - High free triiodothyronine and free-triiodothyronine-to-free-thyroxine ratio levels are associated with metabolic syndrome in a euthyroid population
Diego Urrunaga-Pastor, Mirella Guarnizo-Poma, Enrique Moncada-Mapelli, Luis G. Aguirre, Herbert Lazaro-Alcantara, Socorro Paico-Palacios, Betzi Pantoja-Torres, Vicente A. Benites-Zapata
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2018; 12(2): 155. CrossRef - Exploring the association between thyroid- stimulating hormone and metabolic syndrome: A large population-based study
Yi-Chao Zhou, Wen-Hui Fang, Tung-Wei Kao, Chung-Ching Wang, Yaw-Wen Chang, Tao-Chun Peng, Chen-Jung Wu, Hui-Fang Yang, James Yi-Hsin Chan, Wei-Liang Chen, Tatsuo Shimosawa
PLOS ONE.2018; 13(6): e0199209. CrossRef - Thyroid function and metabolic syndrome in the population-based LifeLines cohort study
Bruce H. R. Wolffenbuttel, Hanneke J. C. M. Wouters, Sandra N. Slagter, Robert P. van Waateringe, Anneke C. Muller Kobold, Jana V. van Vliet-Ostaptchouk, Thera P. Links, Melanie M. van der Klauw
BMC Endocrine Disorders.2017;[Epub] CrossRef - Hormetic effect of triiodothyronine in metabolically healthy obese persons
Ji Eun Jun, Tae Hyuk Kim, Seung-Eun Lee, You-Bin Lee, Jae Hwan Jee, Ji Cheol Bae, Sang-Man Jin, Kyu Yeon Hur, Jae Hyeon Kim, Sun Wook Kim, Jae Hoon Chung, Yong-Ki Min, Moon-Kyu Lee
Endocrine.2017; 57(3): 418. CrossRef - Association of triiodothyronine levels with future development of metabolic syndrome in euthyroid middle-aged subjects: a 6-year retrospective longitudinal study
Hye Jeong Kim, Ji Cheol Bae, Hyeong Kyu Park, Dong Won Byun, Kyoil Suh, Myung Hi Yoo, Jee Jae Hwan, Jae Hyeon Kim, Yong-Ki Min, Sun Wook Kim, Jae Hoon Chung
European Journal of Endocrinology.2017; 176(4): 443. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- The effect of endocrine disrupting chemicals on the vitronectin-receptor (integrin αvβ3)-mediated cell adhesion of human umbilical vein endothelial cells

- Endocrine Research
- Thyroid Hormone Regulates the mRNA Expression of Small Heterodimer Partner through Liver Receptor Homolog-1
- Hwa Young Ahn, Hwan Hee Kim, Ye An Kim, Min Kim, Jung Hun Ohn, Sung Soo Chung, Yoon-Kwang Lee, Do Joon Park, Kyong Soo Park, David D. Moore, Young Joo Park
- Endocrinol Metab. 2015;30(4):584-592. Published online December 31, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.4.584
- 3,751 View
- 39 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Expression of hepatic cholesterol 7α-hydroxylase (CYP7A1) is negatively regulated by orphan nuclear receptor small heterodimer partner (SHP). In this study, we aimed to find whether thyroid hormone regulates SHP expression by modulating the transcriptional activities of liver receptor homolog-1 (LRH-1).
Methods We injected thyroid hormone (triiodothyronine, T3) to C57BL/6J wild type. RNA was isolated from mouse liver and used for microarray analysis and quantitative real-time polymerase chain reaction (PCR). Human hepatoma cell and primary hepatocytes from mouse liver were used to confirm the effect of T3
in vitro . Promoter assay and electrophoretic mobility-shift assay (EMSA) were also performed using human hepatoma cell lineResults Initial microarray results indicated that SHP expression is markedly decreased in livers of T3 treated mice. We confirmed that T3 repressed SHP expression in the liver of mice as well as in mouse primary hepatocytes and human hepatoma cells by real-time PCR analysis. LRH-1 increased the promoter activity of SHP; however, this increased activity was markedly decreased after thyroid hormone receptor β/retinoid X receptor α/T3 administration. EMSA revealed that T3 inhibits specific LRH-1 DNA binding.
Conclusion We found that thyroid hormone regulates the expression of SHP mRNA through interference with the transcription factor, LRH-1.
-
Citations
Citations to this article as recorded by- Bile acid and receptors: biology and drug discovery for nonalcoholic fatty liver disease
Ting-ying Jiao, Yuan-di Ma, Xiao-zhen Guo, Yun-fei Ye, Cen Xie
Acta Pharmacologica Sinica.2022; 43(5): 1103. CrossRef - Loperamide induces excessive accumulation of bile acids in the liver of mice with different diets
Zili Lei, Hedong Rong, Yanhong Yang, Siping Yu, Tianle Zhang, Lei Chen, Ya Nie, Qi Song, Qing Hu, Jiao Guo
Toxicology.2022; 477: 153278. CrossRef - Pathogenesis of hypothyroidism-induced NAFLD is driven by intra- and extrahepatic mechanisms
Giuseppe Ferrandino, Rachel R. Kaspari, Olga Spadaro, Andrea Reyna-Neyra, Rachel J. Perry, Rebecca Cardone, Richard G. Kibbey, Gerald I. Shulman, Vishwa Deep Dixit, Nancy Carrasco
Proceedings of the National Academy of Sciences.2017;[Epub] CrossRef
- Bile acid and receptors: biology and drug discovery for nonalcoholic fatty liver disease

- Thyroid
- Weight Changes in Patients with Differentiated Thyroid Carcinoma during Postoperative Long-Term Follow-up under Thyroid Stimulating Hormone Suppression
- Seo Young Sohn, Ji Young Joung, Yoon Young Cho, Sun Mi Park, Sang Man Jin, Jae Hoon Chung, Sun Wook Kim
- Endocrinol Metab. 2015;30(3):343-351. Published online August 4, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.3.343
- 4,061 View
- 60 Download
- 10 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background There are limited data about whether patients who receive initial treatment for differentiated thyroid cancer (DTC) gain or lose weight during long-term follow-up under thyroid stimulating hormone (TSH) suppression. This study was aimed to evaluate whether DTC patients under TSH suppression experience long-term weight gain after initial treatment. We also examined the impact of the radioactive iodine ablation therapy (RAIT) preparation method on changes of weight, comparing thyroid hormone withdrawal (THW) and recombinant human TSH (rhTSH).
Methods We retrospectively reviewed 700 DTC patients who underwent a total thyroidectomy followed by either RAIT and levothyroxine (T4) replacement or T4 replacement alone. The control group included 350 age-matched patients with benign thyroid nodules followed during same period. Anthropometric data were measured at baseline, 1 to 2 years, and 3 to 4 years after thyroidectomy. Comparisons were made between weight and body mass index (BMI) at baseline and follow-up.
Results Significant gains in weight and BMI were observed 3 to 4 years after initial treatment for female DTC but not in male patients. These gains among female DTC patients were also significant compared to age-matched control. Women in the THW group gained a significant amount of weight and BMI compared to baseline, while there was no increase in weight or BMI in the rhTSH group. There were no changes in weight and BMI in men according to RAIT preparation methods.
Conclusion Female DTC patients showed significant gains in weight and BMI during long-term follow-up after initial treatment. These changes were seen only in patients who underwent THW for RAIT.
-
Citations
Citations to this article as recorded by- Impact of a mobile health intervention based on multi-theory model of health behavior change on self-management in patients with differentiated thyroid cancer: protocol for a randomized controlled trial
Yang Jiang, Xiangju Sun, Maomin Jiang, Hewei Min, Jing Wang, Xinghua Fu, Jiale Qi, Zhenjie Yu, Xiaomei Zhu, Yibo Wu
Frontiers in Public Health.2024;[Epub] CrossRef - Thyroidectomy Effects on the Body Mass Index and Thyroid-Stimulating Hormone: A Systematic Review and Meta-Analysis
Hyder Mirghani, Ahmad M Fnjan, Abdullah F Almalki, Ali F Almadan, Omar Abdullah M Alammar, Abdulaziz S Alhwiati, Amer A Laradhi, Ahmed M Bakour, Mohamad A Aljahed, Abdulmajeed M Alzahrani
Cureus.2024;[Epub] CrossRef - Pre-surgery dietician counseling can prevent post-thyroidectomy body weight gain: results of an intervention trial
Laura Croce, Cristina Pallavicini, Noemi Busca, Benedetto Calì, Giuseppe Bellastella, Francesca Coperchini, Flavia Magri, Luca Chiovato, Hellas Cena, Mario Rotondi
Endocrine.2023; 81(2): 246. CrossRef - Determinants and mediating mechanisms of quality of life and disease-specific symptoms among thyroid cancer patients: the design of the WaTCh study
Floortje Mols, Dounya Schoormans, Romana Netea-Maier, Olga Husson, Sandra Beijer, Katrijn Van Deun, Wouter Zandee, Marleen Kars, Pleun C. M. Wouters van Poppel, Suat Simsek, Patrick van Battum, Jérôme M. H. Kisters, Jan Paul de Boer, Elske Massolt, Rachel
Thyroid Research.2023;[Epub] CrossRef - Effects of a low-iodine diet in post-thyroidectomy thyroid cancer patients undergoing I131 therapy at the Vietnam National Cancer Hospital
Bach Viet Hoang, Tien Thi Hong Nguyen, Yen Thi Duong, Hoa Thi Thanh Nguyen, Thu Ha Nguyen, Thanh Thi Nguyen, Lieu Thi Thu Nguyen, Huong Thi Le
Nutrition and Health.2023;[Epub] CrossRef - Positive effects of thyroid replacement therapy on assisted reproductive technology outcomes in women with subclinical hypothyroidism with positive thyroid peroxidase autoantibodies
Himanshu Arora, Ineabelle Collazo, Katherine L. Palmerola, Madhumita Parmar, Manish Narasimman, Nicholas Hendon, Juergen Eisermann, Maria Bustillo
F&S Reports.2022; 3(1): 32. CrossRef - Weight Gain After Thyroidectomy: A Systematic Review and Meta-Analysis
Christine N Huynh, Janina V Pearce, Le Kang, Francesco S Celi
The Journal of Clinical Endocrinology & Metabolism.2021; 106(1): 282. CrossRef - Weight change in patients with differentiated thyroid carcinoma after total thyroidectomy versus lobectomy
Hae-Ryong Cho, Ra-Yeong Song, Kyung Ho Kang
Korean Journal of Clinical Oncology.2020; 16(2): 127. CrossRef - Postthyroidectomy obesity in a Korean population: does the extent of surgery matter?
Min-Young Park, Sang Eun Nam, Kyoung Sik Park, Madhuri Saindane, Young-Bum Yoo, Jung-Hyun Yang, Ah-Leum Ahn, Jae-Kyung Choi, Won Seo Park
Annals of Surgical Treatment and Research.2019; 97(3): 119. CrossRef - Body weight change is unpredictable after total thyroidectomy
Ron Glick, Paula Chang, Peter Michail, Jonathan W. Serpell, Simon Grodski, James C. Lee
ANZ Journal of Surgery.2018; 88(3): 162. CrossRef - Weight Changes After Thyroid Surgery for Patients with Benign Thyroid Nodules and Thyroid Cancer: Population-Based Study and Systematic Review and Meta-Analysis
Naykky Singh Ospina, Ana Castaneda-Guarderas, Oksana Hamidi, Oscar J. Ponce, Wang Zhen, Larry Prokop, Victor M. Montori, Juan P. Brito
Thyroid.2018; 28(5): 639. CrossRef - Does the Risk of Metabolic Syndrome Increase in Thyroid Cancer Survivors?
Min-Hee Kim, Jin-young Huh, Dong-jun Lim, Moo-Il Kang
Thyroid.2017; 27(7): 936. CrossRef - Thyroid hormone and its metabolites in relation to quality of life in patients treated for differentiated thyroid cancer
E.T. Massolt, M. van der Windt, T.I.M. Korevaar, B.L.R. Kam, J.W. Burger, G.J.H. Franssen, I. Lehmphul, J. Köhrle, W.E. Visser, R.P. Peeters
Clinical Endocrinology.2016; 85(5): 781. CrossRef - High Serum Levels of Thyroid-Stimulating Hormone and Sustained Weight Gain in Patients with Thyroid Cancer Undergoing Radioiodine Therapy
Hyo Jung Seo, June-Key Chung, Keon Wook Kang, E. Edmund Kim, Gi Jeong Cheon, Jin Chul Paeng, Dong Soo Lee, Young Joo Park, Do Joon Park, Jae Gol Choe
International Journal of Thyroidology.2016; 9(1): 19. CrossRef
- Impact of a mobile health intervention based on multi-theory model of health behavior change on self-management in patients with differentiated thyroid cancer: protocol for a randomized controlled trial

- Obesity and Metabolism
- Serum Concentrations of Ghrelin and Leptin according to Thyroid Hormone Condition, and Their Correlations with Insulin Resistance
- Kyu-Jin Kim, Bo-Yeon Kim, Ji-Oh Mok, Chul-Hee Kim, Sung-Koo Kang, Chan-Hee Jung
- Endocrinol Metab. 2015;30(3):318-325. Published online May 18, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.3.318
- 3,837 View
- 45 Download
- 9 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Thyroid hormones can influence energy metabolism and insulin sensitivity via their interaction with adipocytokines and gut hormones. The aims of this study were to evaluate differences in serum ghrelin and leptin concentrations according to thyroid hormone levels, and to investigate the correlation of insulin resistance.
Methods A total of 154 patients (57 hyperthyroid patients, 61 euthyroid patients, and 36 hypothyroid patients; mean age, 47.9 years) were enrolled. Serum leptin, ghrelin, and insulin levels were measured and insulin resistance was calculated using the formula of the homeostasis model assessment of insulin resistance (HOMA-IR).
Results There were no differences in mean concentrations of ghrelin or leptin among the three groups. There were no significant differences in insulin levels between the groups (
P =0.06), although hyperthyroid patients had borderline statistically significantly higher levels of insulin than did euthyroid subjects bypost hoc test (26.4 µIU/mL vs. 16.1 µIU/mL,P =0.057). Regarding HOMA-IR index, the mean levels were highest in the hyperthyroid group among those of the three groups (hyperthyroid vs. euthyroid vs. hypothyroid, 6.7 vs. 3.8 vs. 4.4,P =0.068). Plasma levels of ghrelin were significantly negatively correlated with age, insulin, glucose, body mass index (BMI), and HOMA-IR. Plasma levels of leptin showed significant positive correlation with BMI and triglyceride. There were no significant correlations among thyroid hormone, thyrotropin, ghrelin, leptin, or insulin.Conclusion The present study found that serum ghrelin, leptin, and insulin levels didn't differ according to thyroid function conditions. Further studies with larger numbers of patients are required to establish a direct relationship between plasma ghrelin, leptin, and thyroid hormone.
-
Citations
Citations to this article as recorded by- Insulin resistance, leptin and adiponectin in lean and hypothyroid children and adolescents with obesity
Doaa El Amrousy, Dalia El-Afify, Shaimaa Salah
BMC Pediatrics.2022;[Epub] CrossRef - Mediators of energy homeostasis in hyperthyroidism
Avinash Patil, Suresh Vaikkakara, Mani Deepthi Dasari, Sandeep Ganta, Alok Sachan, Kiranmayi S. Vinapamula
Archives of Endocrinology and Metabolism.2022;[Epub] CrossRef - STUDY OF GHRELIN LEVELS IN HYPOTHYROID PATIENTS BEFORE AND AFTER
TREATMENT
Peeyush Yadav, G. G. Kaushik
INTERNATIONAL JOURNAL OF SCIENTIFIC RESEARCH.2021; : 52. CrossRef - Acylated Ghrelin Attenuates l-Thyroxin–induced Cardiac Damage in Rats by Antioxidant and Anti-inflammatory Effects and Downregulating Components of the Cardiac Renin–angiotensin System
Rehab Badi
Journal of Cardiovascular Pharmacology.2021; 78(3): 422. CrossRef - Experimental hypothyroidism in adult male rats: the effects of Artemisia dracunculus aqueous extract on serum thyroid hormones, lipid profile, leptin, adiponectin, and antioxidant factors
Mohammad Mohsen Mohammadi, Mahdi Saeb, Saeed Nazifi
Comparative Clinical Pathology.2020; 29(2): 485. CrossRef - Leptin, neuropeptide Y (NPY), melatonin and zinc levels in experimental hypothyroidism and hyperthyroidism: relation with melatonin and the pineal gland
Abdulkerim Kasım Baltaci, Rasim Mogulkoc
Hormone Molecular Biology and Clinical Investigation.2018;[Epub] CrossRef - Leptin, NPY, Melatonin and Zinc Levels in Experimental Hypothyroidism and Hyperthyroidism: The Relation to Zinc
Abdulkerim Kasım Baltaci, Rasim Mogulkoc
Biochemical Genetics.2017; 55(3): 223. CrossRef - Thyroid Hormone Regulation and Insulin Resistance: Insights From Animals Naturally Adapted to Fasting
Bridget Martinez, Rudy M. Ortiz
Physiology.2017; 32(2): 141. CrossRef - Role of the Orexin System on the Hypothalamus-Pituitary-Thyroid Axis
Antonietta Messina, Carolina De Fusco, Vincenzo Monda, Maria Esposito, Fiorenzo Moscatelli, Anna Valenzano, Marco Carotenuto, Emanuela Viggiano, Sergio Chieffi, Vincenzo De Luca, Giuseppe Cibelli, Marcellino Monda, Giovanni Messina
Frontiers in Neural Circuits.2016;[Epub] CrossRef - Serum Concentrations of Ghrelin and Leptin according to Thyroid Hormone Condition, and Their Correlations with Insulin Resistance (Endocrinol Metab2015;30:318-25, Kyu-Jin Kim et al.)
Jin Hwa Kim
Endocrinology and Metabolism.2015; 30(4): 631. CrossRef - Serum Concentrations of Ghrelin and Leptin according to Thyroid Hormone Condition, and Their Correlations with Insulin Resistance (Endocrinol Metab2015;30:318-25, Kyu-Jin Kim et al.)
Chan-Hee Jung
Endocrinology and Metabolism.2015; 30(4): 633. CrossRef
- Insulin resistance, leptin and adiponectin in lean and hypothyroid children and adolescents with obesity


 KES
KES

 First
First Prev
Prev



