Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- Efficacy and Safety of Omarigliptin, a Novel Once-Weekly Dipeptidyl Peptidase-4 Inhibitor, in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
- A.B.M. Kamrul-Hasan, Muhammad Shah Alam, Samir Kumar Talukder, Deep Dutta, Shahjada Selim
- Endocrinol Metab. 2024;39(1):109-126. Published online January 23, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1839

- 1,138 View
- 37 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
No recent meta-analysis has holistically analyzed and summarized the efficacy and safety of omarigliptin in type 2 diabetes mellitus (T2DM). We conducted a meta-analysis to address this knowledge gap.
Methods
Electronic databases were searched to identify randomized controlled trials (RCTs) that included patients with T2DM who received omarigliptin in the intervention arm. The control arm consisted of either a placebo (passive control group [PCG]) or an active comparator (active control group [ACG]). The primary outcome assessed was changes in hemoglobin A1c (HbA1c), while secondary outcomes included variations in glucose levels, achievement of glycemic targets, adverse events (AEs), and hypoglycemic events.
Results
From 332 initially screened articles, data from 16 RCTs involving 8,804 subjects were analyzed. Omarigliptin demonstrated superiority over placebo in reducing HbA1c levels (mean difference, –0.58%; 95% confidence interval, –0.75 to –0.40; P<0.00001; I2=91%). Additionally, omarigliptin outperformed placebo in lowering fasting plasma glucose, 2-hour postprandial glucose, and in the percentage of participants achieving HbA1c levels below 7.0% and 6.5%. The glycemic efficacy of omarigliptin was similar to that of the ACG across all measures. Although the omarigliptin group experienced a higher incidence of hypoglycemic events compared to the PCG, the overall AEs, serious AEs, hypoglycemia, and severe hypoglycemia were comparable between the omarigliptin and control groups (PCG and ACG).
Conclusion
Omarigliptin has a favorable glycemic efficacy and safety profile for managing T2DM.

- Calcium & Bone Metabolism
- Real-World Safety and Effectiveness of Denosumab in Patients with Osteoporosis: A Prospective, Observational Study in South Korea
- Yumie Rhee, Dong-Gune Chang, Jeonghoon Ha, Sooa Kim, Yusun Lee, Euna Jo, Jung-Min Koh
- Endocrinol Metab. 2022;37(3):497-505. Published online June 3, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1427

- 5,347 View
- 264 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The efficacy and safety of denosumab have been established in a phase 3, randomized, placebo-controlled trial in Korean postmenopausal women with osteoporosis. This postmarketing surveillance study was aimed to investigate the safety and effectiveness of denosumab in Korean real-world clinical practice.
Methods
Patients with osteoporosis who had received denosumab per the Korean approved indications in the postmarketing setting between September 2014 and September 2019 were enrolled. The primary endpoint was the incidence of adverse events (AEs) and adverse drug reactions (ADRs). The secondary endpoint was the percent change from baseline in bone mineral density (BMD) of the lumbar spine, total hip, and femoral neck.
Results
Of the 3,221 patients enrolled, 3,185 were included in the safety analysis set; 2,973 (93.3%) were female, and the mean± standard deviation (SD) age was 68.9±9.9 years. The mean±SD study period was 350.0±71.4 days. AEs, fatal AEs, and ADRs occurred in 19.3%, 0.8%, and 1.6%, respectively. The most frequent AEs, occurring in >0.5% of patients, were dizziness (0.7%), arthralgia (0.7%), back pain (0.6%), and myalgia (0.6%). Hypocalcemia occurred in 0.3% of patients. There were no cases of osteonecrosis of the jaw and atypical femoral fracture. Mean±SD percent change from baseline in BMD of the lumbar spine, total hip, and femoral neck was 7.3%±23.6%, 3.6%±31.4%, and 3.2%±10.7%, respectively.
Conclusion
The safety and effectiveness of denosumab in Korean patients with osteoporosis in this study were comparable with those in the Korean randomized controlled trial, with no new safety findings. -
Citations
Citations to this article as recorded by- Prevalence of denosumab-induced hypocalcemia: a retrospective observational study of patients routinely monitored with ionized calcium post-injection
Anna Spångeus, Johan Rydetun, Mischa Woisetschläger
Osteoporosis International.2024; 35(1): 173. CrossRef - Cost-consequence analysis of continuous denosumab therapy for osteoporosis treatment in South Korea
Seungju Cha, Minjeong Sohn, Hyowon Yang, Eric J. Yeh, Ki-Hyun Baek, Jeonghoon Ha, Hyemin Ku
BMC Musculoskeletal Disorders.2024;[Epub] CrossRef - Denosumab and the Risk of Diabetes in Patients Treated for Osteoporosis
Huei-Kai Huang, Albert Tzu-Ming Chuang, Tzu-Chi Liao, Shih-Chieh Shao, Peter Pin-Sung Liu, Yu-Kang Tu, Edward Chia-Cheng Lai
JAMA Network Open.2024; 7(2): e2354734. CrossRef - Adverse Effects of Denosumab in Kidney Transplant Recipients: A 20-Year Retrospective Single-Center Observation Study in Central Taiwan
Tsung-Yin Tsai, Zi-Hong You, Shang-Feng Tsai, Ming-Ju Wu, Tung-Min Yu, Ya-Wen Chuang, Yung-Chieh Lin, Ya-Lian Deng, Chiann-Yi Hsu, Cheng-Hsu Chen
Transplantation Proceedings.2023; 55(4): 837. CrossRef - Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study
Chaiho Jeong, Jeongmin Lee, Jinyoung Kim, Jeonghoon Ha, Kwanhoon Jo, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Tae-Seo Sohn, Ki-Ho Song, Moo Il Kang, Ki-Hyun Baek
Endocrinology and Metabolism.2023; 38(2): 260. CrossRef - Effect of Denosumab on Bone Density in Postmenopausal Osteoporosis: A Comparison with and without Calcium Supplementation in Patients on Standard Diets in Korea
Chaiho Jeong, Jinyoung Kim, Jeongmin Lee, Yejee Lim, Dong-Jun Lim, Ki-Hyun Baek, Jeonghoon Ha
Journal of Clinical Medicine.2023; 12(21): 6904. CrossRef - Denosumab
Reactions Weekly.2022; 1919(1): 221. CrossRef - Denosumab, an effective osteoporosis treatment option for men
Sung Hye Kong
The Korean Journal of Internal Medicine.2022; 37(5): 947. CrossRef
- Prevalence of denosumab-induced hypocalcemia: a retrospective observational study of patients routinely monitored with ionized calcium post-injection

- Clinical Study
- Efficacy and Safety of the Novel Dipeptidyl Peptidase-4 Inhibitor Gemigliptin in the Management of Type 2 Diabetes: A Meta-Analysis
- Deep Dutta, Anshita Agarwal, Indira Maisnam, Rajiv Singla, Deepak Khandelwal, Meha Sharma
- Endocrinol Metab. 2021;36(2):374-387. Published online April 6, 2021
- DOI: https://doi.org/10.3803/EnM.2020.818
- 6,369 View
- 224 Download
- 16 Web of Science
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
No meta-analysis has holistically analysed and summarised the efficacy and safety of gemigliptin in type 2 diabetes. The meta-analysis addresses this knowledge gap.
Methods
Electronic databases were searched for randomised controlled trials (RCTs) involving diabetes patients receiving gemigliptin in the intervention arm and placebo/active comparator in the control arm. The primary outcome was change in haemoglobin A1c (HbA1c). The secondary outcomes were alterations in glucose, glycaemic targets, lipids, insulin resistance, and adverse events.
Results
Data from 10 RCTs involving 1,792 patients were analysed. Four had an active control group (ACG), with metformin/dapagliflozin/sitagliptin/glimepiride as the active comparator; six had a passive control group (PCG), with placebo/rosuvastatin as controls. HbA1c reduction by gemigliptin at 24 weeks was comparable to ACG (mean difference [MD], 0.09%; 95% confidence interval [CI], –0.06 to 0.23; P=0.24; I2=0%; moderate certainty of evidence [MCE]), but superior to PCG (MD, –0.91%; 95% CI, –1.18 to –0.63); P<0.01; I2=89%; high certainty of evidence [HCE]). Gemigliptin was superior to PCG regarding achieving HbA1c <7% (12 weeks: odds ratio [OR], 5.91; 95% CI, 1.34 to 26.08; P=0.02; I2=74%; 24 weeks: OR, 4.48; 95% CI, 2.09 to 9.60; P<0.01; I2=69%; HCE). Gemigliptin was comparable to ACG regarding achieving HbA1c <7% after 24 weeks (OR, 0.92; 95% CI, 0.52 to 1.63; P=0.77; I2=66%; MCE). Adverse events were similar between the gemigliptin and control groups (risk ratio [RR], 1.06; 95% CI, 0.82 to 1.36; P=0.66; I2=35%; HCE). The gemigliptin group did not have increased hypoglycaemia (RR, 1.19; 95% CI, 0.62 to 2.28; P=0.61; I2=19%; HCE).
Conclusion
Gemigliptin has good glycaemic efficacy and is well-tolerated over 6 months of use. -
Citations
Citations to this article as recorded by- Hyperprolactinemia Due to Prolactinoma has an Adverse Impact on Bone Health with Predominant Impact on Trabecular Bone: A Systematic Review and Meta-Analysis
Lakshmi Nagendra, Deep Dutta, Sunetra Mondal, Nitin Kapoor, Ameya Joshi, Saptarshi Bhattacharya
Journal of Clinical Densitometry.2024; 27(1): 101453. CrossRef - Impact of early initiation of ezetimibe in patients with acute coronary syndrome: A systematic review and meta-analysis
Kunal Mahajan, Lakshmi Nagendra, Anil Dhall, Deep Dutta
European Journal of Internal Medicine.2024;[Epub] CrossRef - Efficacy and safety of dorzagliatin, a novel glucokinase activators, in the treatment of T2DM: A meta-analysis of randomized controlled trials
Yuqian Wu, Kai Wang, Jingyang Su, Xin Liu
Medicine.2024; 103(8): e36916. CrossRef - Glucagon-Like Peptide-1 Receptor Agonists in Post-bariatric Surgery Patients: A Systematic Review and Meta-analysis
Deep Dutta, Lakshmi Nagendra, Ameya Joshi, Suryashri Krishnasamy, Meha Sharma, Naresh Parajuli
Obesity Surgery.2024; 34(5): 1653. CrossRef - Orforglipron, a novel non‐peptide oral daily glucagon‐like peptide‐1 receptor agonist as an anti‐obesity medicine: A systematic review and meta‐analysis
Deep Dutta, Lakshmi Nagendra, Beatrice Anne, Manoj Kumar, Meha Sharma, A. B. M. Kamrul‐Hasan
Obesity Science & Practice.2024;[Epub] CrossRef - Efficacy and safety of novel dual glucokinase activator dorzagliatin in type-2 diabetes A meta-analysis
Deep Dutta, Deepak Khandelwal, Manoj Kumar, Meha Sharma
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102695. CrossRef - Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis
Deep Dutta, Saptarshi Bhattacharya, Manoj Kumar, Priyankar K. Datta, Ritin Mohindra, Meha Sharma
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102697. CrossRef - Effects of Initial Combinations of Gemigliptin Plus Metformin Compared with Glimepiride Plus Metformin on Gut Microbiota and Glucose Regulation in Obese Patients with Type 2 Diabetes: The INTESTINE Study
Soo Lim, Minji Sohn, Jose C. Florez, Michael A. Nauck, Jiyoung Ahn
Nutrients.2023; 15(1): 248. CrossRef - Systematic review and meta-analysis of teneligliptin for treatment of type 2 diabetes
R. Pelluri, S. Kongara, V. R. Nagasubramanian, S. Mahadevan, J. Chimakurthy
Journal of Endocrinological Investigation.2023; 46(5): 855. CrossRef - Efficacy and safety of enavogliflozin versus dapagliflozin added to metformin plus gemigliptin treatment in patients with type 2 diabetes: A double-blind, randomized, comparator-active study: ENHANCE-D study
Kyung-Soo Kim, Kyung Ah Han, Tae Nyun Kim, Cheol-Young Park, Jung Hwan Park, Sang Yong Kim, Yong Hyun Kim, Kee Ho Song, Eun Seok Kang, Chul Sik Kim, Gwanpyo Koh, Jun Goo Kang, Mi Kyung Kim, Ji Min Han, Nan Hee Kim, Ji Oh Mok, Jae Hyuk Lee, Soo Lim, Sang S
Diabetes & Metabolism.2023; 49(4): 101440. CrossRef - Verapamil improves One-Year C-Peptide Levels in Recent Onset Type-1 Diabetes: A Meta-Analysis
Deep Dutta, Lakshmi Nagendra, Nishant Raizada, Saptarshi Bhattacharya, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2023; 27(3): 192. CrossRef - Role of novel sodium glucose co-transporter-2 inhibitor enavogliflozin in type-2 diabetes: A systematic review and meta-analysis
Deep Dutta, B.G. Harish, Beatrice Anne, Lakshmi Nagendra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(8): 102816. CrossRef - Semaglutide and cancer: A systematic review and meta-analysis
Lakshmi Nagendra, Harish BG, Meha Sharma, Deep Dutta
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(9): 102834. CrossRef - Efficacy and Safety of Novel Thiazolidinedione Rivoglitazone in Type-2 Diabetes a Meta-Analysis
Deep Dutta, Jyoti Kadian, Indira Maisnam, Ashok Kumar, Saptarshi Bhattacharya, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2023; 27(4): 286. CrossRef - Impact of early initiation of proprotein convertase subtilisin/kexin type 9 inhibitors in patients with acute coronary syndrome: A systematic review meta-analysis
Lakshmi Nagendra, Kunal Mahajan, Gunjan Gupta, Deep Dutta
Indian Heart Journal.2023; 75(6): 416. CrossRef - Optimal use of once weekly icodec insulin in type-2 diabetes: An updated meta-analysis of phase-2 and phase-3 randomized controlled trials
Deep Dutta, Lakshmi Nagendra, Sowrabha Bhat, Ritin Mohindra, Vineet Surana, Anoop Misra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(10): 102877. CrossRef - Impact of Enhanced External Counter-pulsation Therapy on Glycaemic Control in People With Prediabetes and Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis
Lakshmi Nagendra, Deep Dutta, Meha Sharma, Harish Bg
touchREVIEWS in Endocrinology.2023; 19(2): 8. CrossRef - Role of Novel Glucagon-like Peptide-1 Receptor Analogue Polyethylene Glycol Loxenatide in Type 2 Diabetes: A Systematic Review and Meta-analysis
Deep Dutta, Subhankar Chatterjee, Priyankar K. Datta, Ritin Mohindra, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2023; 27(5): 377. CrossRef - Efficacy and Safety of Ultra-rapid Lispro Insulin in Managing Type-1 and Type-2 Diabetes: A Systematic Review and Meta-Analysis
Deep Dutta, Lakshmi Nagendra, Saptarshi Bhattacharya, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2023; 27(6): 467. CrossRef - Safety and efficacy of once weekly dipeptidyl-peptidase-4 inhibitor trelagliptin in type-2 diabetes: A meta-analysis
Deep Dutta, Ritin Mohindra, Vineet Surana, Meha Sharma
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2022; 16(4): 102469. CrossRef - Efficacy and safety of hydroxychloroquine for managing glycemia in type-2 diabetes: A systematic review and meta-analysis
D Dutta, R Jindal, D Mehta, M Kumar, M Sharma
Journal of Postgraduate Medicine.2022; 68(2): 85. CrossRef - Gemigliptin exerts protective effects against doxorubicin-induced hepatotoxicity by inhibiting apoptosis via the regulation of fibroblast growth factor 21 expression
Kyeong-Min Lee, Yeo Jin Hwang, Gwon-Soo Jung
Biochemical and Biophysical Research Communications.2022; 626: 135. CrossRef - Reporting and methodological quality of systematic reviews of DPP-4 inhibitors for patients with type 2 diabetes mellitus: an evidence-based mapping
Zouxi Du, Tingting Lu, Mingdong Gao, Limin Tian
Acta Diabetologica.2022; 59(12): 1539. CrossRef - Ranirestat improves electrophysiologic but not clinical measures of diabetic polyneuropathy: A meta-analysis
Deep Dutta, Ritin Mohindra, Manoj Kumar, Ashok Kumar, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2022; 26(5): 399. CrossRef - Efficacy and safety of novel twincretin tirzepatide a dual GIP and GLP-1 receptor agonist in the management of type-2 diabetes: A Cochrane meta-analysis
Deep Dutta, Vineet Surana, Rajiv Singla, Sameer Aggarwal, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2021; 25(6): 475. CrossRef
- Hyperprolactinemia Due to Prolactinoma has an Adverse Impact on Bone Health with Predominant Impact on Trabecular Bone: A Systematic Review and Meta-Analysis

- Clinical Study
- Efficacy and Safety of Pitavastatin in a Real-World Setting: Observational Study Evaluating SaFety in Patient Treated with Pitavastatin in Korea (PROOF Study)
- In-Kyung Jeong, Sung-Rae Kim
- Endocrinol Metab. 2020;35(4):882-891. Published online December 2, 2020
- DOI: https://doi.org/10.3803/EnM.2020.723
- 5,606 View
- 248 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
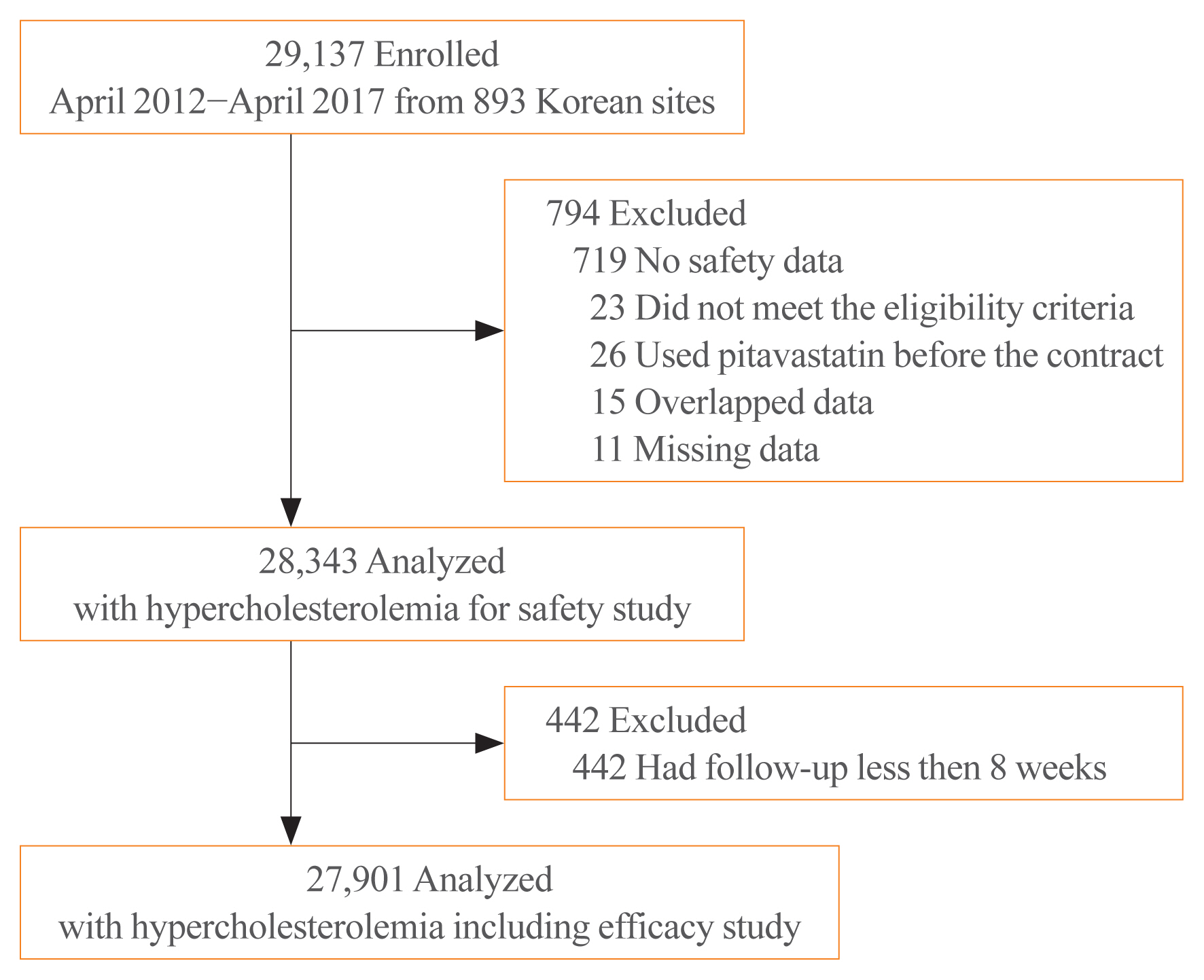
While randomized controlled trials provide useful information about drug safety and efficacy, they do not always reflect the observed results in the real world. The prospective, observational, non-comparative trial in South Korea was designed to evaluate the efficacy and safety of pitavastatin in clinical practice in 28,343 patients.
Methods
This study was conducted in 893 facilities in Korea from April 2, 2012 to April 1, 2017. This study was designed to administer 1, 2, or 4 mg pitavastatin to patients with hyperlipidemia at the age of 20 or older for at least 8 weeks.
Results
For 126 days of mean duration of administration of pitavastatin, the % change of low density lipoprotein cholesterol indicated a dose dependent reduction: –23.4%, –29.1%, and –35.2% in the 1, 2, and 4 mg groups, respectively in patients who have not been treated with lipid lowering medications prior to study. Only 1.74% (492/28,343) of pitavastatin-treated patients experienced adverse events, of which 0.43% (123/28,343) were adverse drug reactions. Less than 1% of patients experienced the grade 2 or more toxicity (Common Terminology Criteria for Adverse Events v4.03) in alanine aminotransferase, aspartate aminotransferase, serum creatinine, and serum creatine phosphokinase. Although there were no rhabdomyolysis in 28,343 patients, 0.04% of patients had been reported pitavastatin-related musculoskeletal disorders.
Conclusion
Overall, this observational study showed that pitavastatin was well tolerated and effectively modified the lipid profile, reducing cardiovascular and cerebrovascular risk in Korean patients with hypercholesterolemia in the real world. -
Citations
Citations to this article as recorded by- Low-Density Lipoprotein Cholesterol Level, Statin Use and Myocardial Infarction Risk in Young Adults
Heekyoung Jeong, Kyungdo Han, Soon Jib Yoo, Mee Kyoung Kim
Journal of Lipid and Atherosclerosis.2022; 11(3): 288. CrossRef
- Low-Density Lipoprotein Cholesterol Level, Statin Use and Myocardial Infarction Risk in Young Adults

- Clinical Study
- Revisiting Rupture of Benign Thyroid Nodules after Radiofrequency Ablation: Various Types and Imaging Features
- Sae Rom Chung, Jung Hwan Baek, Jin Yong Sung, Ji Hwa Ryu, So Lyung Jung
- Endocrinol Metab. 2019;34(4):415-421. Published online December 23, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.4.415
- 5,786 View
- 96 Download
- 22 Web of Science
- 24 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background To evaluate the imaging features, clinical manifestations, and prognosis of patients with thyroid nodule rupture after radiofrequency ablation (RFA).
Methods The records of 12 patients who experienced thyroid nodule rupture after RFA at four Korean thyroid centers between March 2010 and July 2017 were retrospectively reviewed. Clinical data evaluated included baseline patient characteristics, treatment methods, initial presenting symptoms, imaging features, treatment, and prognosis.
Results The most common symptoms of post-RFA nodule rupture were sudden neck bulging and pain. Based on imaging features, the localization of nodule rupture was classified into three types: anterior, posterolateral, and medial types. The anterior type is the most often, followed by posterolateral and medial type. Eight patients recovered completely after conservative treatment. Four patients who did not improve with conservative management required invasive procedures, including incision and drainage or aspiration.
Conclusion Thyroid nodule rupture after RFA can be classified into three types based on its localization: anterior, posterolateral, and medial types. Because majority of thyroid nodule ruptures after RFA can be managed conservatively, familiarity with these imaging features is essential in avoiding unnecessary imaging workup or invasive procedures.
-
Citations
Citations to this article as recorded by- Assessing the efficacy of thyroid nodule radiofrequency ablation using patient-reported outcome measures
Ege Akgun, Gustavo Romero-Velez, Eren Berber
Surgery.2024; 175(3): 654. CrossRef - The Comparison of Efficacy and Safety between Radiofrequency Ablation Alone and Ethanol Ablation Followed by Radiofrequency Ablation in the Treatment of Mixed Cystic and Solid Thyroid Nodule
Min Gang Jo, Min Kyoung Lee, Jae Ho Shin, Min Guk Seo, So Lyung Jung
Journal of the Korean Society of Radiology.2024;[Epub] CrossRef - Cutaneous fistula formation after thyroid nodule rupture: A rare complication after radiofrequency ablation
Amanda J. Bastien, Luv Amin, Jeffrey Moses, Wendy Sacks, Allen S. Ho
Head & Neck.2024;[Epub] CrossRef - Thyroid nodule rupture after radiofrequency ablation: case report and literature review
Tatiana Ferraro, Sameeha Sajid, Steven P. Hodak, Chelsey K. Baldwin
Frontiers in Endocrinology.2024;[Epub] CrossRef - Radiofrequency Ablation for Benign Thyroid Nodules: Radiology In Training
Ningcheng Li, Timothy C. Huber
Radiology.2023; 306(1): 54. CrossRef - A Case of Thyroid Abscess Following Ethanol Ablation for Benign Thyroid Nodule
Heungrae Cho, Dongbin Ahn, Ji Hye Kwak, Gil Joon Lee
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2023; 66(9): 624. CrossRef - Radiofrequency Ablation for Benign Thyroid Nodules
Julia E Noel, Catherine F Sinclair
The Journal of Clinical Endocrinology & Metabolism.2023; 109(1): e12. CrossRef - 2022 Taiwan clinical multicenter expert consensus and recommendations for thyroid radiofrequency ablation
Wei-Che Lin, Wen-Chieh Chen, Pei-Wen Wang, Yi-Chia Chan, Yen-Hsiang Chang, Harn-Shen Chen, Szu-Tah Chen, Wei-Chih Chen, Kai-Lun Cheng, Shun-Yu Chi, Pi-Ling Chiang, Chen-Kai Chou, Feng-Fu Chou, Shun-Chen Huang, Feng-Hsuan Liu, Sheng-Dean Luo, Fen-Yu Tseng,
Ultrasonography.2023; 42(3): 357. CrossRef - Effective and Safe Application of Radiofrequency Ablation for Benign Thyroid Nodules
Jin Yong Sung
Journal of the Korean Society of Radiology.2023; 84(5): 985. CrossRef - General Principles for the Safe Performance, Training, and Adoption of Ablation Techniques for Benign Thyroid Nodules: An American Thyroid Association Statement
Catherine F. Sinclair, Jung Hwan Baek, Kathleen E. Hands, Steven P. Hodak, Timothy C. Huber, Iram Hussain, Brian Hung-Hin Lang, Julia E. Noel, Maria Papaleontiou, Kepal N. Patel, Gilles Russ, Jonathon Russell, Stefano Spiezia, Jennifer H. Kuo
Thyroid®.2023; 33(10): 1150. CrossRef - Radiofrequency ablation and thyroid cancer: review of the current literature
Haris Muhammad, Aniqa Tehreem, Jonathon O. Russell
American Journal of Otolaryngology.2022; 43(1): 103204. CrossRef - Microwave Ablation Vs Traditional Thyroidectomy for Benign Thyroid Nodules: A Prospective, Non-Randomized Cohort Study
Shaokun Li, Mingfeng Yang, Haipeng Guo, Muyuan Liu, Shaowei Xu, Hanwei Peng
Academic Radiology.2022; 29(6): 871. CrossRef - Radiofrequency ablation and related ultrasound‐guided ablation technologies for treatment of benign and malignant thyroid disease: An international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with
Lisa A. Orloff, Julia E. Noel, Brendan C. Stack, Marika D. Russell, Peter Angelos, Jung Hwan Baek, Kevin T. Brumund, Feng‐Yu Chiang, Mary Beth Cunnane, Louise Davies, Andrea Frasoldati, Anne Y. Feng, Laszlo Hegedüs, Ayaka J. Iwata, Emad Kandil, Jennifer K
Head & Neck.2022; 44(3): 633. CrossRef - Thyroid Nodule Radiofrequency Ablation: Complications and Clinical Follow Up
James Y. Lim, Jennifer H. Kuo
Techniques in Vascular and Interventional Radiology.2022; 25(2): 100824. CrossRef - Minimally-invasive treatments for benign thyroid nodules: recommendations for information to patients and referring physicians by the Italian Minimally-Invasive Treatments of the Thyroid group
Giovanni Mauri, Stella Bernardi, Andrea Palermo, Roberto Cesareo, Enrico Papini, Luigi Solbiati, Daniele Barbaro, Salvatore Monti, Maurilio Deandrea, Laura Fugazzola, Giovanni Gambelunghe, Roberto Negro, Stefano Spiezia, Fulvio Stacul, Luca Maria Sconfien
Endocrine.2022; 76(1): 1. CrossRef - American Association of Clinical Endocrinology Disease State Clinical Review: The Clinical Utility of Minimally Invasive Interventional Procedures in the Management of Benign and Malignant Thyroid Lesions
Sina Jasim, Kepal N. Patel, Gregory Randolph, Stephanie Adams, Roberto Cesareo, Edward Condon, Tara Henrichsen, Malak Itani, Maria Papaleontiou, Leonardo Rangel, John Schmitz, Marius N. Stan
Endocrine Practice.2022; 28(4): 433. CrossRef - Radiofrequency Ablation of Benign and Malignant Thyroid Nodules
Patrick J. Navin, Scott M. Thompson, Anil N. Kurup, Robert A. Lee, Matthew R. Callstrom, M. Regina Castro, Marius N. Stan, Brian T. Welch, John J. Schmitz
RadioGraphics.2022; 42(6): 1812. CrossRef - SFE-AFCE-SFMN 2022 consensus on the management of thyroid nodules: Thermal ablation
Adrien Ben Hamou, Edouard Ghanassia, Arnaud Muller, Miriam Ladsous, Nunzia Cinzia Paladino, Laurent Brunaud, Laurence Leenhardt, Gilles Russ
Annales d'Endocrinologie.2022; 83(6): 423. CrossRef - Complications of RFA for Thyroid Nodules: Prevention and Management
Rahul K. Sharma, Jennifer H Kuo
Current Otorhinolaryngology Reports.2021; 9(1): 79. CrossRef - Ultrasonographic characteristics of thyroid nodule rupture after microwave ablation
Peng Tian, Wenyan Du, Xiaoxi Liu, Yiwen Ding, Zekai Zhang, Jing Li, Yanzhen Wang
Medicine.2021; 100(9): e25070. CrossRef - Symptomatic aseptic necrosis of benign thyroid lesions after microwave ablation: risk factors and clinical significance
Jian-ping Dou, Jie Yu, Zhi-gang Cheng, Fang-yi Liu, Xiao-ling Yu, Qi-di Hou, Fang Liu, Zhi-yu Han, Ping Liang
International Journal of Hyperthermia.2021; 38(1): 815. CrossRef - The Importance of Nodule Size in the Management of Ruptured Thyroid Nodule After Radiofrequency Ablation: A Retrospective Study and Literature Review
Wen-Chieh Chen, Sheng-Dean Luo, Wei-Chih Chen, Chen-Kai Chou, Yen-Hsiang Chang, Kai-Lun Cheng, Wei-Che Lin
Frontiers in Endocrinology.2021;[Epub] CrossRef - Long-Term Follow-Up of Single-Fiber Multiple Low-Intensity Energy Laser Ablation Technique of Benign Thyroid Nodules
Mattia Squarcia, Mireia Mora, Gloria Aranda, Enrique Carrero, Daniel Martínez, Ramona Jerez, Ricard Valero, Joan Berenguer, Irene Halperin, Felicia A. Hanzu
Frontiers in Oncology.2021;[Epub] CrossRef - Effectiveness of Injecting Cold 5% Dextrose into Patients with Nerve Damage Symptoms during Thyroid Radiofrequency Ablation
Min Kyoung Lee, Jung Hwan Baek, Sae Rom Chung, Young Jun Choi, Yu-Mi Lee, Tae Yong Kim, Jeong Hyun Lee
Endocrinology and Metabolism.2020; 35(2): 407. CrossRef
- Assessing the efficacy of thyroid nodule radiofrequency ablation using patient-reported outcome measures

- Thyroid
- A Comparison of Ultrasound-Guided Fine Needle Aspiration versus Core Needle Biopsy for Thyroid Nodules: Pain, Tolerability, and Complications
- Eun Ji Jeong, Sae Rom Chung, Jung Hwan Baek, Young Jun Choi, Jae Kyun Kim, Jeong Hyun Lee
- Endocrinol Metab. 2018;33(1):114-120. Published online March 21, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.1.114
- 5,369 View
- 61 Download
- 24 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background To compare pain, tolerability, and complications associated with fine needle aspiration (FNA) versus core needle biopsy (CNB).
Methods FNAs were performed using 23-gauge needles and CNBs were performed using 18-gauge double-action spring-activated needles in 100 patients for each procedure. Patients were asked to record a pain score using a 10-cm visual analog scale and procedure tolerability. Complications and number of biopsies were recorded.
Results The median pain scores were similar for the FNA and CNB approaches during and 20 minutes after the biopsy procedures (3.7 vs. 3.6,
P =0.454; 0.9 vs. 1.1,P =0.296, respectively). The procedure was tolerable in all 100 FNA patients and in 97 CNB patients (P =0.246). The mean number of biopsies was fewer in the CNB group (1.4 vs. 1.2,P =0.002). By subgroup analysis (staff vs. non-staff), no significant difference was detected in any parameter. There were no major complications in either group, but three patients who underwent CNB had minor complications (P =0.246).Conclusion FNA and CNB show no significant differences for diagnosing thyroid nodules in terms of pain, tolerability, or complications.
-
Citations
Citations to this article as recorded by- A comparative analysis of core needle biopsy and repeat fine needle aspiration in patients with inconclusive initial cytology of thyroid nodules
Xuejiao Su, Can Yue, Wanting Yang, Buyun Ma
Frontiers in Endocrinology.2024;[Epub] CrossRef - Assessing Adequacy: A Meta-Analysis of Rapid Onsite Evaluation of Thyroid Nodules
Peter P. Issa, Christina McCarthy, Mohammad Hussein, Aaron L. Albuck, Essam Emad, Mohamed Shama, Krzysztof Moroz, Eman Toraih, Emad Kandil
Journal of Surgical Research.2024; 296: 523. CrossRef - Histology-based and cytology-based needle sampling for targeted next-generation sequencing in the indeterminate thyroid tumors
Chun-Nan Chen, Tsung-Lin Yang
European Archives of Oto-Rhino-Laryngology.2023; 280(8): 3773. CrossRef - Preoperative evaluation of thyroid nodules – Diagnosis and management strategies
Tapoi Dana Antonia, Lambrescu Ioana Maria, Gheorghisan-Galateanu Ancuta-Augustina
Pathology - Research and Practice.2023; 246: 154516. CrossRef - Keloid Development After Fine Needle Aspiration of the Thyroid: A Rare Case and Review of Management Strategies
Shaniah S Holder, Alaerebo S Malvan-iyalla, Sara Arfan, Vimal Basani, Frederick Tiesenga
Cureus.2023;[Epub] CrossRef - Utilidad de la biopsia con aguja gruesa ecoguiada en nódulos tiroideos con punción aspirativa con aguja fina no diagnóstica
R. Cortázar-García, M.D. Martín-Escalante, L. Robles-Cabeza, C. Martínez-Santos
Radiología.2022; 64(3): 195. CrossRef - Thyroid diagnostic modalities (fine needle aspiration and core needle biopsy) with histology correlation: a tertiary centre experience
Sona J Appukutty, Anna Paterson, Nishant S Patel, Adam Duckworth, James Chan, Maria O'Donovan, Alison J Marker
Journal of Clinical Pathology.2022; 75(9): 620. CrossRef - Diagnostic performance of core needle biopsy for nodal recurrences in patients with head and neck squamous cell carcinoma
Ta-Hsuan Lo, Cheng-Ping Wang, Chun-Nan Chen, Tsung-Lin Yang, Pei-Jen Lou, Jenq-Yuh Ko, Yih-Leong Chang, Tseng-Cheng Chen
Scientific Reports.2022;[Epub] CrossRef - The efficacy of incorporating ultrasound-guided core biopsy into the clinical workflow of indeterminate thyroid tumors
Chun-Nan Chen, Min-Shu Hsieh, Yi-Hsuan Lee, Tsung-Lin Yang
Journal of the Formosan Medical Association.2022; 121(10): 2012. CrossRef - A Literature Review of Factors Associated With Pain From Fine Needle Aspiration Biopsy of Thyroid Nodules
Tao Liu, Manisha Tilak, Sara Awad, Joshua Lakoff
Endocrine Practice.2022; 28(6): 628. CrossRef - Usefulness of ultrasound-guided core biopsy in thyroid nodules with inconclusive fine-needle aspiration biopsy findings
R. Cortázar-García, M.D. Martín-Escalante, L. Robles-Cabeza, C. Martínez-Santos
Radiología (English Edition).2022; 64(3): 195. CrossRef - Permanent vocal fold paralysis after ultrasound-guided core needle biopsy of thyroid nodule
Kathrin Zimmerman, Matthew Hoffman, Amalee Smith, C. Blake Simpson
Otolaryngology Case Reports.2022; 24: 100455. CrossRef - Interobserver variability in ultrasound assessment of thyroid nodules
Jaber Alyami, Fahad F. Almutairi, Sultan Aldoassary, Amani Albeshry, Ali Almontashri, Mazen Abounassif, Majed Alamri
Medicine.2022; 101(41): e31106. CrossRef - Usage and Diagnostic Yield of Fine-Needle Aspiration Cytology and Core Needle Biopsy in Thyroid Nodules: A Systematic Review and Meta-Analysis of Literature Published by Korean Authors
Soon-Hyun Ahn
Clinical and Experimental Otorhinolaryngology.2021; 14(1): 116. CrossRef - Hydrodissection: A Novel Approach for Safe Core Needle Biopsy of Small High-Risk Subcapsular Thyroid Nodules
Hojat Ebrahiminik, Hossein Chegeni, Javad Jalili, Rambod Salouti, Hadi Rokni, Afshin Mohammadi, Ali Mosaddegh Khah, Seyed Mohammad Tavangar, Zahra Ebrahiminik
CardioVascular and Interventional Radiology.2021; 44(10): 1651. CrossRef - A Blinded Randomized Trial Comparing 2 Needle Gauges for Fine‐Needle Biopsy of Thyroid Nodules
Christopher M. Shumrick, Jonathan C. Simmonds, Lorna L. Ogden, Cindi A. Snowden, Jagdish K. Dhingra
OTO Open.2021;[Epub] CrossRef - Application of biomarkers in the diagnosis of uncertain samples of core needle biopsy of thyroid nodules
Yan Xiong, Xin Li, Li Liang, Dong Li, Limin Yan, Xueying Li, Jiting Di, Ting Li
Virchows Archiv.2021; 479(5): 961. CrossRef - Diagnostic Efficacy and Safety of Core Needle Biopsy as a First-Line Diagnostic Method for Thyroid Nodules: A Prospective Cohort Study
Min Ji Hong, Dong Gyu Na, Hunkyung Lee
Thyroid.2020; 30(8): 1141. CrossRef - Comparison Between Fine Needle Aspiration and Core Needle Biopsy for the Diagnosis of Thyroid Nodules: Effective Indications According to US Findings
Soo Yeon Hahn, Jung Hee Shin, Young Lyun Oh, Ko Woon Park, Yaeji Lim
Scientific Reports.2020;[Epub] CrossRef - 2019 Practice guidelines for thyroid core needle biopsy: a report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association
Chan Kwon Jung, Jung Hwan Baek, Dong Gyu Na, Young Lyun Oh, Ka Hee Yi, Ho-Cheol Kang
Journal of Pathology and Translational Medicine.2020; 54(1): 64. CrossRef - Pathological diagnosis of thyroid nodules based on core needle biopsies: comparative study between core needle biopsies and resected specimens in 578 cases
Yan Xiong, Limin Yan, Lin Nong, Yalin Zheng, Ting Li
Diagnostic Pathology.2019;[Epub] CrossRef - The Significance of Having an Excellent Patient's Comfort with Thyroid Core Needle Biopsy
Pierpaolo Trimboli, Luca Giovanella
Endocrinology and Metabolism.2018; 33(1): 53. CrossRef
- A comparative analysis of core needle biopsy and repeat fine needle aspiration in patients with inconclusive initial cytology of thyroid nodules


 KES
KES

 First
First Prev
Prev



