Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
- Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
- Joon Ho Moon, Kyuho Kim, Sung Hee Choi
- Endocrinol Metab. 2022;37(4):575-586. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.402
- 7,724 View
- 434 Download
- 11 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
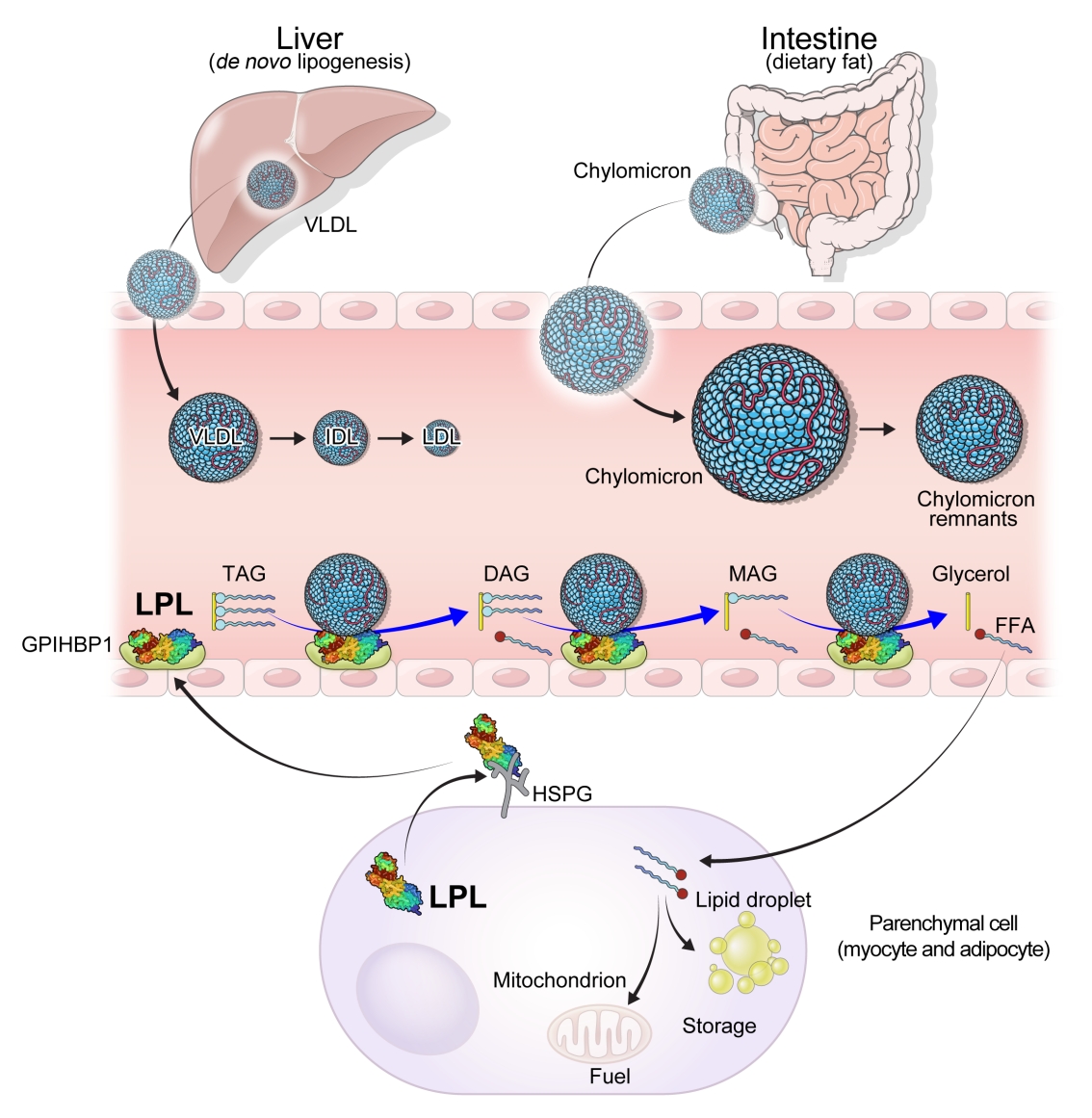
ePub - High levels of triglycerides (TG) and triglyceride-rich lipoproteins (TGRLs) confer a residual risk of cardiovascular disease after optimal low-density lipoprotein cholesterol (LDL-C)–lowering therapy. Consensus has been made that LDL-C is a non-arguable primary target for lipid lowering treatment, but the optimization of TGRL for reducing the remnant risk of cardiovascular diseases is urged. Omega-3 fatty acids and fibrates are used to reduce TG levels, but many patients still have high TG and TGRL levels combined with low high-density lipoprotein concentration that need to be ideally treated. Lipoprotein lipase (LPL) is a key regulator for TGs that hydrolyzes TGs to glycerol and free fatty acids in lipoprotein particles for lipid storage and consumption in peripheral organs. A deeper understanding of human genetics has enabled the identification of proteins regulating the LPL activity, which include the apolipoproteins and angiopoietin-like families. Novel therapeutic approach such as antisense oligonucleotides and monoclonal antibodies that regulate TGs have been developed in recent decades. In this article, we focus on the biology of LPL and its modulators and review recent clinical application, including genetic studies and clinical trials of novel therapeutics. Optimization of LPL activity to lower TG levels could eventually reduce incident atherosclerotic cardiovascular disease in conjunction with successful LDL-C reduction.
-
Citations
Citations to this article as recorded by- The chylomicron saga: time to focus on postprandial metabolism
Alejandro Gugliucci
Frontiers in Endocrinology.2024;[Epub] CrossRef - Sanghuangporus vaninii extract ameliorates hyperlipidemia in rats by mechanisms identified with transcriptome analysis
Ning Gao, Yuanzhen Liu, Guangjie Liu, Bo Liu, Yupeng Cheng
Food Science & Nutrition.2024;[Epub] CrossRef - Targeting host-specific metabolic pathways—opportunities and challenges for anti-infective therapy
Monika I. Konaklieva, Balbina J. Plotkin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the obesity medicine association and the National Lipid Association 2024
Harold Edward Bays, Carol Kirkpatrick, Kevin C. Maki, Peter P. Toth, Ryan T. Morgan, Justin Tondt, Sandra Michelle Christensen, Dave Dixon, Terry A. Jacobson
Obesity Pillars.2024; : 100108. CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Xanthohumol, a prenylated chalcone, regulates lipid metabolism by modulating the LXRα/RXR-ANGPTL3-LPL axis in hepatic cell lines and high-fat diet-fed zebrafish models
Wan-Yun Gao, Pei-Yi Chen, Hao-Jen Hsu, Je-Wen Liou, Chia-Ling Wu, Ming-Jiuan Wu, Jui-Hung Yen
Biomedicine & Pharmacotherapy.2024; 174: 116598. CrossRef - High producer variant of lipoprotein lipase may protect from hepatocellular carcinoma in alcohol-associated cirrhosis
Franziska Schmalz, Janett Fischer, Hamish Innes, Stephan Buch, Christine Möller, Madlen Matz-Soja, Witigo von Schönfels, Benjamin Krämer, Bettina Langhans, Alexandra Klüners, Michael Soyka, Felix Stickel, Jacob Nattermann, Christian P. Strassburg, Thomas
JHEP Reports.2023; 5(4): 100684. CrossRef - Measurement of Serum Low Density Lipoprotein Cholesterol and Triglyceride-Rich Remnant Cholesterol as Independent Predictors of Atherosclerotic Cardiovascular Disease: Possibilities and Limitations
Dieter Lütjohann, Hans-Ulrich Klör, Frans Stellaard
Nutrients.2023; 15(9): 2202. CrossRef - Influence of antipsychotic medications on hyperlipidemia risk in patients with schizophrenia: evidence from a population-based cohort study and in vitro hepatic lipid homeostasis gene expression
Tien-Yuan Wu, Ni Tien, Cheng-Li Lin, Yu-Cun Cheah, Chung Y. Hsu, Fuu-Jen Tsai, Yi-Jen Fang, Yun-Ping Lim
Frontiers in Medicine.2023;[Epub] CrossRef - Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(13): 4399. CrossRef - Sugar and Dyslipidemia: A Double-Hit, Perfect Storm
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(17): 5660. CrossRef - Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview
Sang Heon Suh, Soo Wan Kim
Diabetes & Metabolism Journal.2023; 47(5): 612. CrossRef - Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease
Elena Valeria Fuior, Evangelia Zvintzou, Theodosios Filippatos, Katerina Giannatou, Victoria Mparnia, Maya Simionescu, Anca Violeta Gafencu, Kyriakos E. Kypreos
Biomedicines.2023; 11(10): 2696. CrossRef - Developing a model to predict the early risk of hypertriglyceridemia based on inhibiting lipoprotein lipase (LPL): a translational study
Julia Hernandez-Baixauli, Gertruda Chomiciute, Juan María Alcaide-Hidalgo, Anna Crescenti, Laura Baselga-Escudero, Hector Palacios-Jordan, Elisabet Foguet-Romero, Anna Pedret, Rosa M. Valls, Rosa Solà, Miquel Mulero, Josep M. Del Bas
Scientific Reports.2023;[Epub] CrossRef
- The chylomicron saga: time to focus on postprandial metabolism

- A Novel Cytosolic Isoform of Mitochondrial Trans-2-Enoyl-CoA Reductase Enhances Peroxisome Proliferator-Activated Receptor α Activity
- Dong-Gyu Kim, Jae Cheal Yoo, Eunju Kim, Young-Sun Lee, Oleg V. Yarishkin, Da Yong Lee, Kun Ho Lee, Seong-Geun Hong, Eun Mi Hwang, Jae-Yong Park
- Endocrinol Metab. 2014;29(2):185-194. Published online June 26, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.2.185
- 4,240 View
- 45 Download
- 20 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Mitochondrial trans-2-enoyl-CoA reductase (MECR) is involved in mitochondrial synthesis of fatty acids and is highly expressed in mitochondria. MECR is also known as nuclear receptor binding factor-1, which was originally reported with yeast two-hybrid screening as a binding protein of the nuclear hormone receptor peroxisome proliferator-activated receptor α (PPARα). However, MECR and PPARα are localized at different compartment, mitochondria, and the nucleus, respectively. Therefore, the presence of a cytosolic or nuclear isoform of MECR is necessary for functional interaction between MECR and PPARα.
Methods To identify the expression pattern of MECR and the cytosolic form of MECR (cMECR), we performed reverse transcription polymerase chain reaction (RT-PCR) with various tissue samples from Sprague-Dawley rats. To confirm the interaction between cMECR and PPARα, we performed several binding assays such as yeast two-hybrid, coimmunoprecipitation, and bimolecular fluorescence complementation. To observe subcellular localization of these proteins, immunocytochemistry was performed. A luciferase assay was used to measure PPARα activity.
Results We provide evidence of an alternatively spliced variant of the rat MECR gene that yields cMECR. The cMECR lacks the N-terminal 76 amino acids of MECR and shows uniform distribution in the cytoplasm and nucleus of HeLa cells. cMECR directly bound PPARα in the nucleus and increased PPARα-dependent luciferase activity in HeLa cells.
Conclusion We found the cytosolic form of MECR (cMECR) was expressed in the cytosolic and/or nuclear region, directly binds with PPARα, and enhances PPARα activity.
-
Citations
Citations to this article as recorded by- Metabolism of phenolics in coffee and plant-based foods by canonical pathways: an assessment of the role of fatty acid β-oxidation to generate biologically-active and -inactive intermediates
Michael N. Clifford, Laurence J. King, Asimina Kerimi, Maria Gema Pereira-Caro, Gary Williamson
Critical Reviews in Food Science and Nutrition.2024; 64(11): 3326. CrossRef - Comparison of muscle nutritional composition, texture quality, carotenoid metabolites and transcriptome to underling muscle quality difference between wild-caught and pond-cultured Yellow River carp (Cyprinus carpio haematopterus)
Luming Wang, Jinrui Xiong, Chunchu Xu, Chaobin Qin, Yuru Zhang, Liping Yang, Shaoyang Zhi, Jianxin Feng, Guoxing Nie
Aquaculture.2024; 581: 740392. CrossRef - Effects of microcystin-LR on immune function, lipid metabolism and intestinal microbial structure in Eriocheir sinensis
Jinliang Du, Liping Cao, Jiancao Gao, Zhijuan Nie, Quanjie Li, Yi Sun, Nailin Shao, Jiawen Hu, Lin Zhou, Guojun Yin, Gangchun Xu
Aquaculture Reports.2024; 35: 101994. CrossRef - A defect in mitochondrial fatty acid synthesis impairs iron metabolism and causes elevated ceramide levels
Debdeep Dutta, Oguz Kanca, Seul Kee Byeon, Paul C. Marcogliese, Zhongyuan Zuo, Rishi V. Shridharan, Jun Hyoung Park, Guang Lin, Ming Ge, Gali Heimer, Jennefer N. Kohler, Matthew T. Wheeler, Benny A. Kaipparettu, Akhilesh Pandey, Hugo J. Bellen
Nature Metabolism.2023; 5(9): 1595. CrossRef - Alternative splicing liberates a cryptic cytoplasmic isoform of mitochondrial MECR that antagonizes influenza virus
Steven F. Baker, Helene Meistermann, Manuel Tzouros, Aaron Baker, Sabrina Golling, Juliane Siebourg Polster, Mitchell P. Ledwith, Anthony Gitter, Angelique Augustin, Hassan Javanbakht, Andrew Mehle, Frank Kirchhoff
PLOS Biology.2022; 20(12): e3001934. CrossRef - Genetic variants in ALDH1L1 and GLDC influence the serine-to-glycine ratio in Hispanic children
Sergey A Krupenko, Shelley A Cole, Ruixue Hou, Karin Haack, Sandra Laston, Nitesh R Mehta, Anthony G Comuzzie, Nancy F Butte, V Saroja Voruganti
The American Journal of Clinical Nutrition.2022; 116(2): 500. CrossRef - Simultaneous Presentation of Multiple Myeloma and Lung Cancer: Case Report and Gene Bioinformatics Analysis
Ping-Ping Xiao, Bing-Qing Luo, Wei Fan, Xu-Yan Chen, Zhi-Gao Dong, Jin-Mei Huang, Yi Zhang, Yong-Quan Chen
Frontiers in Oncology.2022;[Epub] CrossRef - Fatty acid metabolism-related genes are associated with flavor-presenting aldehydes in Chinese local chicken
Xiaoya Yuan, Huanxian Cui, Yuxi Jin, Wenjuan Zhao, Xiaojing Liu, Yongli Wang, Jiqiang Ding, Li Liu, Jie Wen, Guiping Zhao
Frontiers in Genetics.2022;[Epub] CrossRef - NRBF2-mediated autophagy contributes to metabolite replenishment and radioresistance in glioblastoma
Jeongha Kim, Hyunkoo Kang, Beomseok Son, Min-Jung Kim, JiHoon Kang, Kang Hyun Park, Jaewan Jeon, Sunmi Jo, Hae Yu Kim, HyeSook Youn, BuHyun Youn
Experimental & Molecular Medicine.2022; 54(11): 1872. CrossRef - Mitochondrial Fatty Acids and Neurodegenerative Disorders
Alexander J. Kastaniotis, Kaija J. Autio, Remya R. Nair
The Neuroscientist.2021; 27(2): 143. CrossRef - The effects of chronic cadmium exposure on Bufo gargarizans larvae: Histopathological impairment, gene expression alteration and fatty acid metabolism disorder in the liver
Zongqi Ju, Jing Ya, Xinyi Li, Hongyuan Wang, Hongfeng Zhao
Aquatic Toxicology.2020; 222: 105470. CrossRef - Exploration of targets regulated by miR-125b in porcine adipocytes
Xiao Cheng, Xingping Chen, Peng Wang, Ting Chen, Jiajie Sun, Qianyun Xi, Yongliang Zhang
In Vitro Cellular & Developmental Biology - Animal.2020; 56(2): 103. CrossRef - Mitochondrial fatty acid synthesis coordinates oxidative metabolism in mammalian mitochondria
Sara M Nowinski, Ashley Solmonson, Scott F Rusin, J Alan Maschek, Claire L Bensard, Sarah Fogarty, Mi-Young Jeong, Sandra Lettlova, Jordan A Berg, Jeffrey T Morgan, Yeyun Ouyang, Bradley C Naylor, Joao A Paulo, Katsuhiko Funai, James E Cox, Steven P Gygi,
eLife.2020;[Epub] CrossRef - Polymorphisms in ten candidate genes are associated with conformational and locomotive traits in Spanish Purebred horses
Natalia Sevane, Susana Dunner, Ana Boado, Javier Cañon
Journal of Applied Genetics.2017; 58(3): 355. CrossRef - Deep RNA sequencing of pectoralis muscle transcriptomes during late-term embryonic to neonatal development in indigenous Chinese duck breeds
Chunhong Zhu, Weitao Song, Zhiyun Tao, Hongxiang Liu, Wenjuan Xu, Shuangjie Zhang, Huifang Li, Cristina Óvilo
PLOS ONE.2017; 12(8): e0180403. CrossRef - Mitochondrial fatty acid synthesis, fatty acids and mitochondrial physiology
Alexander J. Kastaniotis, Kaija J. Autio, Juha M. Kerätär, Geoffray Monteuuis, Anne M. Mäkelä, Remya R. Nair, Laura P. Pietikäinen, Antonina Shvetsova, Zhijun Chen, J. Kalervo Hiltunen
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2017; 1862(1): 39. CrossRef - Genetic modifications of Mecr reveal a role for mitochondrial 2-enoyl-CoA/ACP reductase in placental development in mice
Remya R. Nair, Juha M. Kerätär, Kaija J. Autio, Ali J. Masud, Mikko A.J. Finnilä, Helena I. Autio-Harmainen, Ilkka J. Miinalainen, Pentti A. Nieminen, J. Kalervo Hiltunen, Alexander J. Kastaniotis
Human Molecular Genetics.2017; 26(11): 2104. CrossRef - MECR Mutations Cause Childhood-Onset Dystonia and Optic Atrophy, a Mitochondrial Fatty Acid Synthesis Disorder
Gali Heimer, Juha M. Kerätär, Lisa G. Riley, Shanti Balasubramaniam, Eran Eyal, Laura P. Pietikäinen, J. Kalervo Hiltunen, Dina Marek-Yagel, Jeffrey Hamada, Allison Gregory, Caleb Rogers, Penelope Hogarth, Martha A. Nance, Nechama Shalva, Alvit Veber, Mic
The American Journal of Human Genetics.2016; 99(6): 1229. CrossRef - Genome‐wide association study with the risk of schizophrenia in a Korean population
Lyoung Hyo Kim, Byung Lae Park, Hyun Sub Cheong, Suhg Namgoong, Ji On Kim, Jeong‐Hyun Kim, Joong‐Gon Shin, Chul Soo Park, Bong‐Jo Kim, Jae Won Kim, Ihn‐Geun Choi, Jaeuk Hwang, Hyoung Doo Shin, Sung‐Il Woo
American Journal of Medical Genetics Part B: Neuropsychiatric Genetics.2016; 171(2): 257. CrossRef - A global transcriptional analysis of Megalobrama amblycephala revealing the molecular determinants of diet-induced hepatic steatosis
Dingdong Zhang, Kangle Lu, Guangzhen Jiang, Wenbin Liu, Zaijie Dong, Hongyan Tian, Xiangfei Li
Gene.2015; 570(2): 255. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef
- Metabolism of phenolics in coffee and plant-based foods by canonical pathways: an assessment of the role of fatty acid β-oxidation to generate biologically-active and -inactive intermediates


 KES
KES

 First
First Prev
Prev



