Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- The Emerging Importance of Mitochondria in White Adipocytes: Neither Last nor Least
- Juan Cai, Fenfen Wang, Mengle Shao
- Endocrinol Metab. 2023;38(5):493-503. Published online October 10, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1813
- 1,926 View
- 92 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
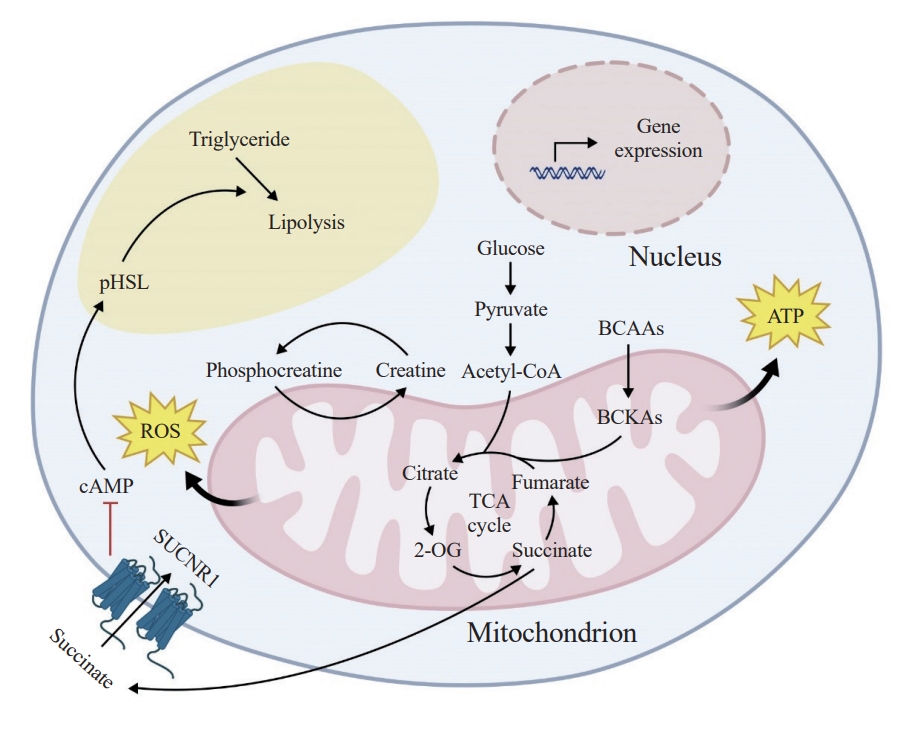
ePub - The growing recognition of mitochondria’s crucial role in the regulation of white adipose tissue remodeling and energy balance underscores its significance. The marked metabolic diversity of mitochondria provides the molecular and cellular foundation for enabling adipose tissue plasticity in response to various metabolic cues. Effective control of mitochondrial function at the cellular level, not only in thermogenic brown and beige adipocytes but also in energy-storing white adipocytes, exerts a profound influence on adipose homeostasis. Furthermore, mitochondria play a pivotal role in intercellular communication within adipose tissue via production of metabolites with signaling properties. A more comprehensive understanding of mitochondrial regulation within white adipocytes will empower the development of targeted and efficacious strategies to enhance adipose function, leading to advancements in overall metabolic health.

- Diabetes, Obesity and Metabolism
- Gemigliptin Alleviates Succinate-Induced Hepatic Stellate Cell Activation by Ameliorating Mitochondrial Dysfunction
- Giang Nguyen, So Young Park, Dinh Vinh Do, Dae-Hee Choi, Eun-Hee Cho
- Endocrinol Metab. 2022;37(6):918-928. Published online November 15, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1530
- 3,450 View
- 229 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
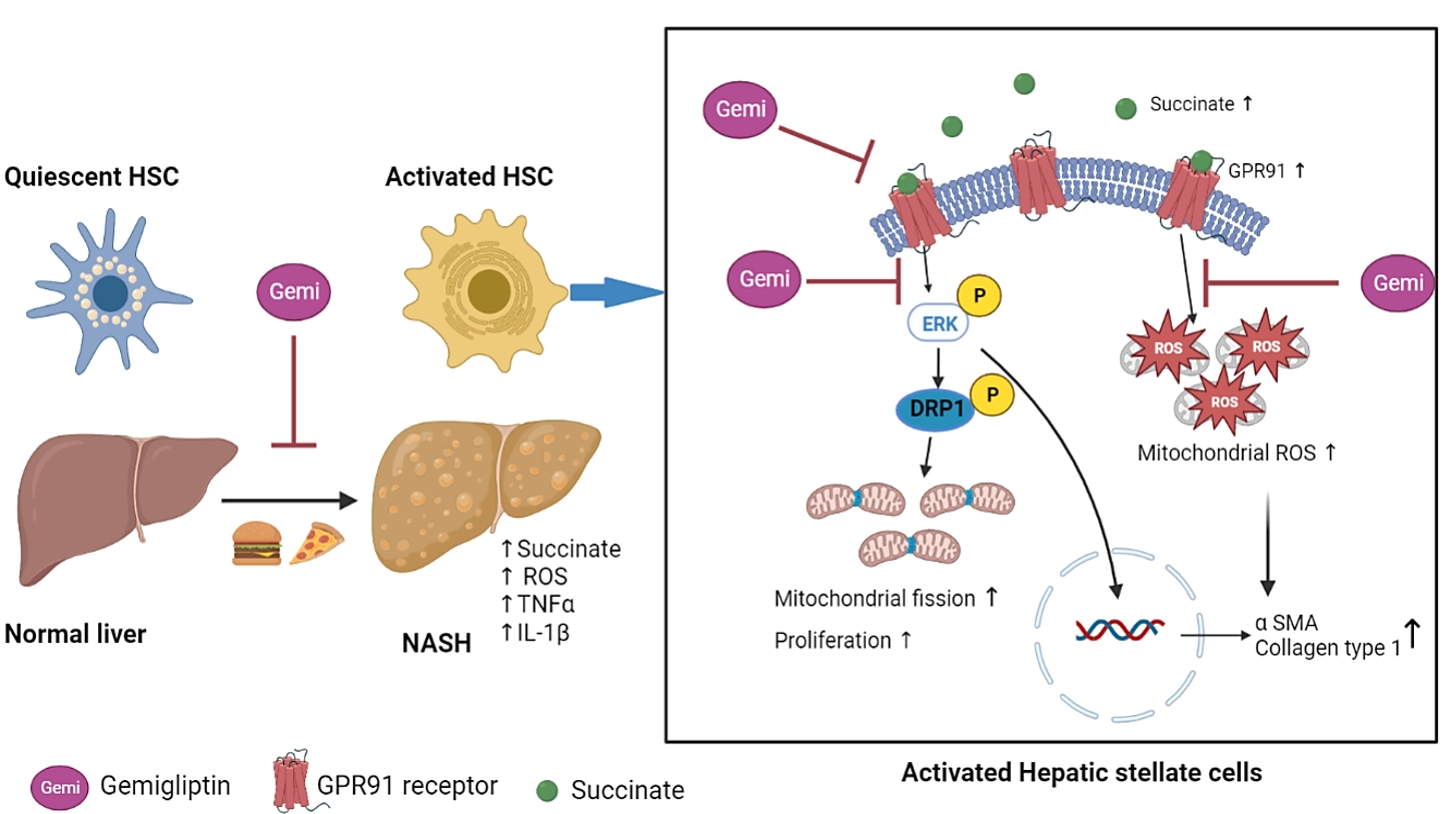
ePub - Background
Dipeptidyl peptidase-4 inhibitors (DPP-4Is) are used clinically as oral antidiabetic agents. Although DPP-4Is are known to ameliorate liver fibrosis, the protective mechanism of DPP-4Is in liver fibrosis remains obscure. In this study, gemigliptin was used to investigate the potential of DPP-4Is to alleviate the progression of liver fibrosis.
Methods
To clarify the effects and mechanisms of gemigliptin, we conducted various experiments in LX-2 cells (immortalized human hepatic stellate cells [HSCs], the principal effectors of hepatic fibrogenesis), which were activated by succinate and exhibited elevated expression of α-smooth muscle actin, collagen type 1, and pro-inflammatory cytokines and increased cell proliferation. In vivo, we examined the effects and mechanisms of gemigliptin on a high-fat, high-cholesterol–induced mouse model of nonalcoholic steatohepatitis (NASH).
Results
Gemigliptin decreased the expression of fibrogenesis markers and reduced the abnormal proliferation of HSCs. In addition, gemigliptin reduced the succinate-induced production of mitochondrial reactive oxygen species (ROS), intracellular ROS, and mitochondrial fission in HSCs. Furthermore, in the mouse model of NASH-induced liver fibrosis, gemigliptin alleviated both liver fibrosis and mitochondrial dysfunction.
Conclusion
Gemigliptin protected against HSC activation and liver fibrosis by alleviating mitochondrial dysfunction and ROS production, indicating its potential as a strategy for preventing the development of liver disease. -
Citations
Citations to this article as recorded by- Improvement effect of gemigliptin on salivary gland dysfunction in exogenous methylglyoxal-injected rats
Woo Kwon Jung, Su-Bin Park, Hwa Young Yu, Junghyun Kim
Heliyon.2024; 10(8): e29362. CrossRef - Gemigliptin, a DPP4 inhibitor, ameliorates nonalcoholic steatohepatitis through AMP-activated protein kinase-independent and ULK1-mediated autophagy
Youngmi Song, Hyekyung Yang, Juhee Kim, Yoonjin Lee, Sung-Ho Kim, In-Gu Do, Cheol-Young Park
Molecular Metabolism.2023; 78: 101806. CrossRef - DPP-4 Inhibitor in Type 2 Diabetes Mellitus Patient with Non-Alcoholic Fatty Liver Disease: Achieving Two Goals at Once?
Ji Cheol Bae
Endocrinology and Metabolism.2022; 37(6): 858. CrossRef
- Improvement effect of gemigliptin on salivary gland dysfunction in exogenous methylglyoxal-injected rats

- Miscellaneous
- Clinical Value of Serum Mitochondria-Inhibiting Substances in Assessing Renal Hazards: A Community-Based Prospective Study in Korea
- Hoon Sung Choi, Jin Taek Kim, Hong Kyu Lee, Wook Ha Park, Youngmi Kim Pak, Sung Woo Lee
- Endocrinol Metab. 2021;36(6):1298-1306. Published online November 26, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1226
- 3,254 View
- 95 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
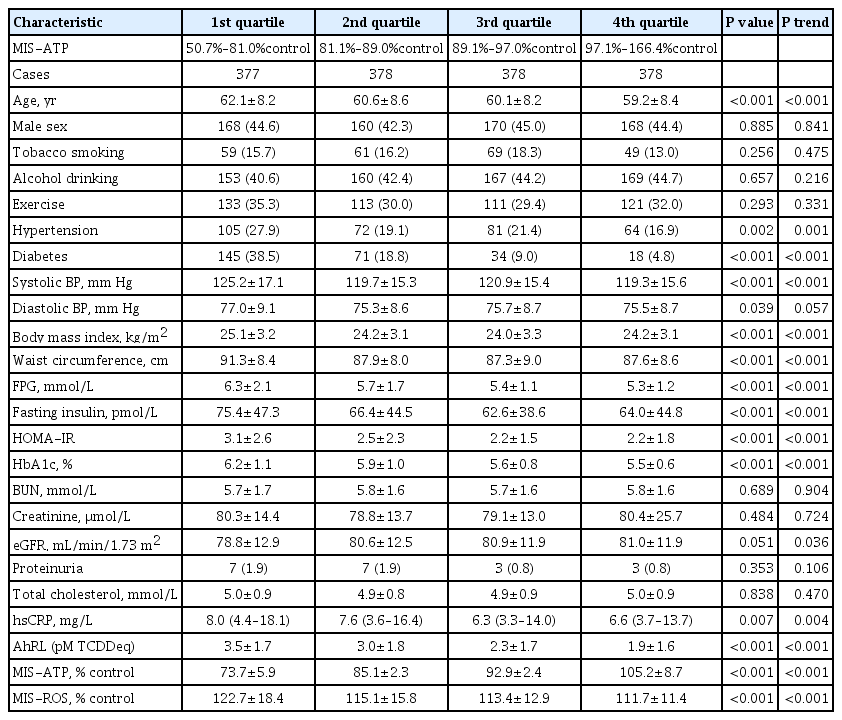
ePub - Background
Mitochondrial dysfunction is strongly associated with several kidney diseases. However, no studies have evaluated the potential renal hazards of serum mitochondria-inhibiting substance (MIS) and aryl hydrocarbon receptor ligand (AhRL) levels.
Methods
We used serum level of MIS and AhRL and clinical renal outcomes from 1,511 participants of a prospective community-based cohort in Ansung. MIS was evaluated based on intracellular adenosine triphosphate (MIS-ATP) or reactive oxygen species (MIS-ROS) generation measured using cell-based assays.
Results
During a mean 6.9-year follow-up, 84 participants (5.6%) developed a rapid decline in kidney function. In the lowest quartile group of MIS-ATP, patients were older and had metabolically deleterious parameters. In multivariate logistic regression analysis, higher MIS-ATP was associated with decreased odds for rapid decline: the odds ratio (OR) of 1% increase was 0.977 (95% confidence interval [CI], 0.957 to 0.998; P=0.031), while higher MIS-ROS was marginally associated with increased odds for rapid decline (OR, 1.014; 95% CI, 0.999 to 1.028; P=0.055). However, serum AhRL was not associated with the rapid decline in kidney function. In subgroup analysis, the renal hazard of MIS was particularly evident in people with hypertension and low baseline kidney function.
Conclusion
Serum MIS was independently associated with a rapid decline in kidney function, while serum AhRL was not. The clinical implication of renal hazard on serum MIS requires further evaluation in future studies. -
Citations
Citations to this article as recorded by- An Interactive Online App for Predicting Diabetes via Machine Learning from Environment-Polluting Chemical Exposure Data
Rosy Oh, Hong Kyu Lee, Youngmi Kim Pak, Man-Suk Oh
International Journal of Environmental Research and Public Health.2022; 19(10): 5800. CrossRef
- An Interactive Online App for Predicting Diabetes via Machine Learning from Environment-Polluting Chemical Exposure Data

- Obesity and Metabolism
- Cellular and Intercellular Homeostasis in Adipose Tissue with Mitochondria-Specific Stress
- Min Jeong Choi, Saet-Byel Jung, Joon Young Chang, Minho Shong
- Endocrinol Metab. 2021;36(1):1-11. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2021.956
- 5,458 View
- 227 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
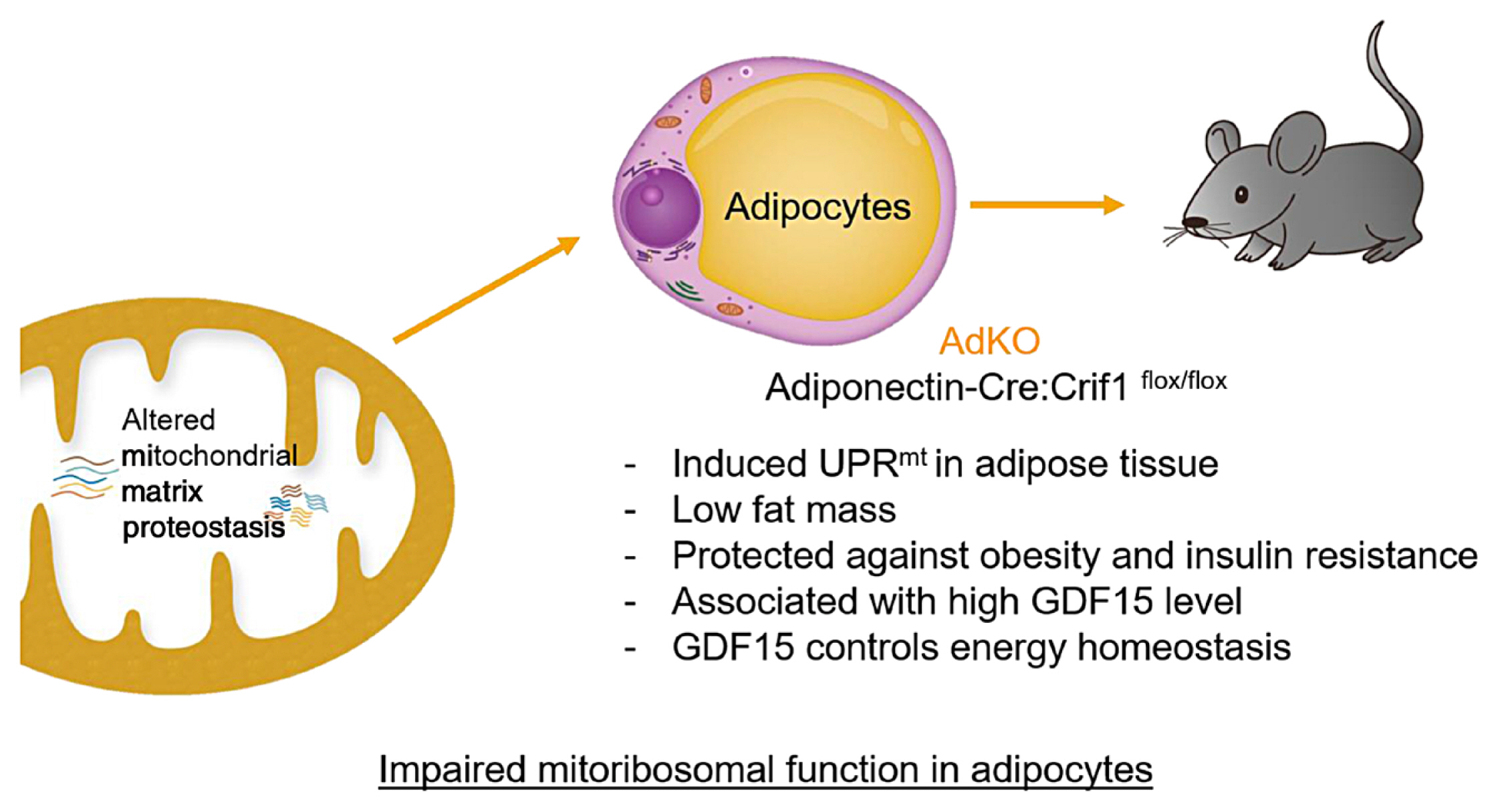
ePub - Paracrine interactions are imperative for the maintenance of adipose tissue intercellular homeostasis, and intracellular organelle dysfunction results in local and systemic alterations in metabolic homeostasis. It is currently accepted that mitochondrial proteotoxic stress activates the mitochondrial unfolded protein response (UPRmt) in vitro and in vivo. The induction of mitochondrial chaperones and proteases during the UPRmt is a key cell-autonomous mechanism of mitochondrial quality control. The UPRmt also affects systemic metabolism through the secretion of cell non-autonomous peptides and cytokines (hereafter, metabokines). Mitochondrial function in adipose tissue plays a pivotal role in whole-body metabolism and human diseases. Despite continuing interest in the role of the UPRmt and quality control pathways of mitochondria in energy metabolism, studies on the roles of the UPRmt and metabokines in white adipose tissue are relatively sparse. Here, we describe the role of the UPRmt in adipose tissue, including adipocytes and resident macrophages, and the interactive roles of cell non-autonomous metabokines, particularly growth differentiation factor 15, in local adipose cellular homeostasis and systemic energy metabolism.
-
Citations
Citations to this article as recorded by- Mitochondrial stress-induced GFRAL signaling controls diurnal food intake and anxiety-like behavior
Carla Igual Gil, Bethany M Coull, Wenke Jonas, Rachel N Lippert, Susanne Klaus, Mario Ost
Life Science Alliance.2022; 5(11): e202201495. CrossRef - Stress-induced FGF21 and GDF15 in obesity and obesity resistance
Susanne Keipert, Mario Ost
Trends in Endocrinology & Metabolism.2021; 32(11): 904. CrossRef
- Mitochondrial stress-induced GFRAL signaling controls diurnal food intake and anxiety-like behavior

- Miscellaneous
- Sarcopenia and Muscle Aging: A Brief Overview
- Tam Dao, Alexander E. Green, Yun A Kim, Sung-Jin Bae, Ki-Tae Ha, Karim Gariani, Mi-ra Lee, Keir J. Menzies, Dongryeol Ryu
- Endocrinol Metab. 2020;35(4):716-732. Published online December 23, 2020
- DOI: https://doi.org/10.3803/EnM.2020.405
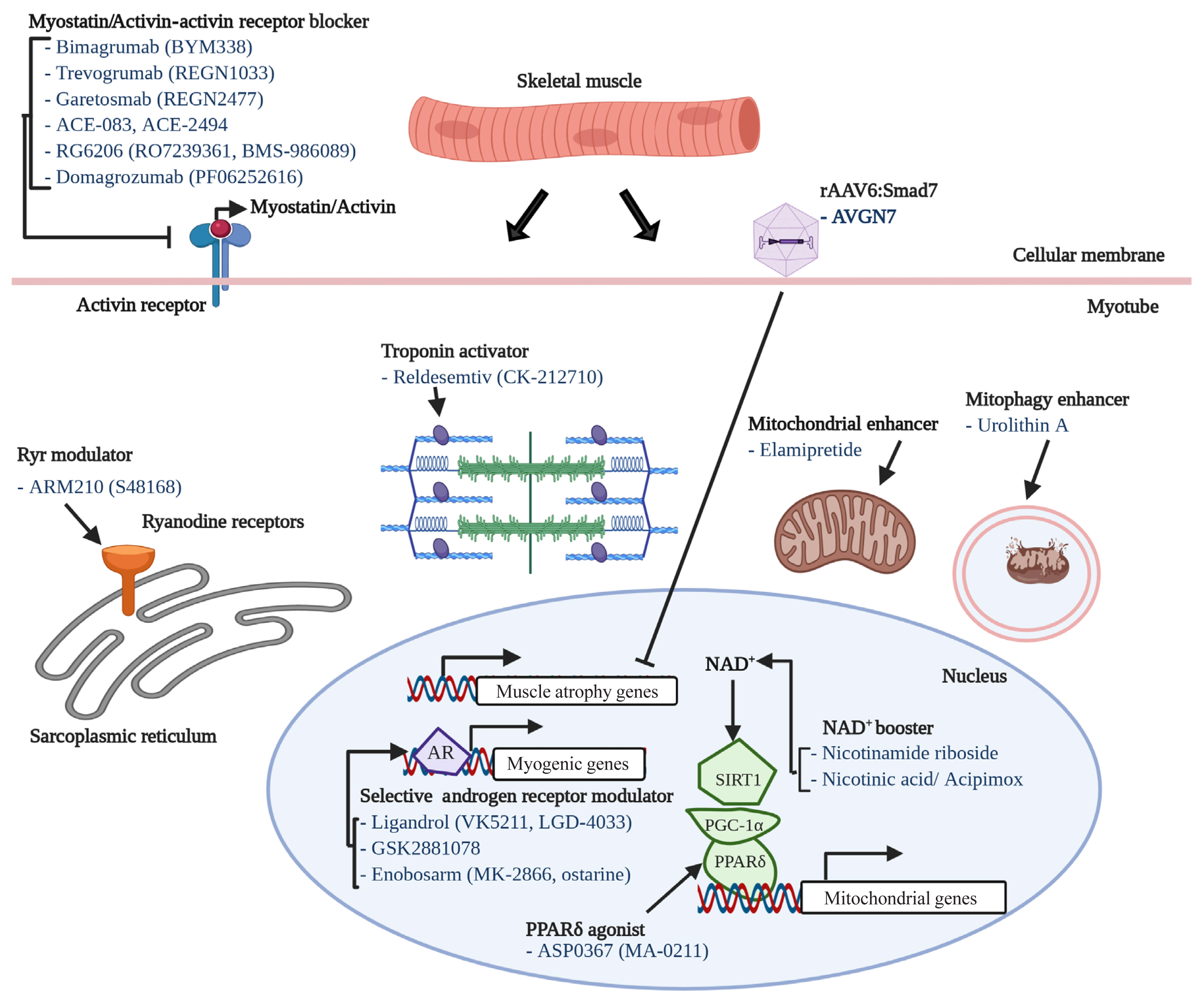
- 22,717 View
- 1,264 Download
- 74 Web of Science
- 79 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The world is facing the new challenges of an aging population, and understanding the process of aging has therefore become one of the most important global concerns. Sarcopenia is a condition which is defined by the gradual loss of skeletal muscle mass and function with age. In research and clinical practice, sarcopenia is recognized as a component of geriatric disease and is a current target for drug development. In this review we define this condition and provide an overview of current therapeutic approaches. We further highlight recent findings that describe key pathophysiological phenotypes of this condition, including alterations in muscle fiber types, mitochondrial function, nicotinamide adenine dinucleotide (NAD+) metabolism, myokines, and gut microbiota, in aged muscle compared to young muscle or healthy aged muscle. The last part of this review examines new therapeutic avenues for promising treatment targets. There is still no accepted therapy for sarcopenia in humans. Here we provide a brief review of the current state of research derived from various mouse models or human samples that provide novel routes for the development of effective therapeutics to maintain muscle health during aging.
-
Citations
Citations to this article as recorded by- Interactions between mitochondrial dysfunction and other hallmarks of aging: Paving a path toward interventions that promote healthy old age
Yuan Li, Laura Berliocchi, Zhiquan Li, Lene Juel Rasmussen
Aging Cell.2024;[Epub] CrossRef - Circulating lumican as a potential biomarker for osteosarcopenia in older adults
So Jeong Park, Eunhye Ji, Hyun Ju Yoo, Kyunggon Kim, Sunghwan Ji, Ji Yeon Baek, Jin Young Lee, Hee-Won Jung, Il-Young Jang, Eunju Lee, Namki Hong, Beom-Jun Kim
Bone.2024; 179: 116959. CrossRef - D‐galactose might mediate some of the skeletal muscle hypertrophy‐promoting effects of milk—A nutrient to consider for sarcopenia?
Jan Homolak, Ana Babic Perhoc, Davor Virag, Ana Knezovic, Jelena Osmanovic Barilar, Melita Salkovic‐Petrisic
BioEssays.2024;[Epub] CrossRef - Dark tea extract attenuates age-related muscle loss by suppressing oxidative stress and inflammation in skeletal muscle of mice
Ahyoung Yoo, Hyo Deok Seo, Jeong-Hoon Hahm, Chang Hwa Jung, Jiyun Ahn, Tae Youl Ha
Journal of Functional Foods.2024; 112: 105980. CrossRef - Moderate dietary restriction delays the onset of age-associated sarcopenia in Caenorhabditis elegans due to reduced myosin UNC-54 degradation
Sobha Tumbapo, Adam Strudwick, Jana J. Stastna, Simon C. Harvey, Marieke J. Bloemink
Mechanisms of Ageing and Development.2024; 217: 111900. CrossRef - Trop T, hand grip strength and waist circumference as markers of sarcopenic obesity in postmenopausal women: An analytical cross-sectional study
Sheetal Sarangi, Arul Senghor K. A., Vinodhini V. M.
Indian Journal of Physiology and Pharmacology.2024; 68: 57. CrossRef - Temporal Muscle Thickness: A Practical Approximation for Assessing Muscle Mass in Older Adults
Miguel German Borda, Jonathan Patricio Baldera, Jessica Samuelsson, Anna Zettergren, Lina Rydén, Eric Westman, Mario Ulises Pérez-Zepeda, Silke Kern, Luis Carlos Venegas, Gustavo Duque, Ingmar Skoog, Dag Aarsland
Journal of the American Medical Directors Association.2024; 25(4): 664. CrossRef - Undernutrition in obese older adults by fat percentage
Meris Esra Bozkurt, Tugba Erdogan, Nezahat Muge Catikkas, Serdar Ozkok, Cihan Kilic, Gulistan Bahat, Mehmet Akif Karan
Aging Clinical and Experimental Research.2024;[Epub] CrossRef - The BET inhibitor JQ1 targets fat metabolism and counteracts obesity
Claudia Fornelli, Alessia Sofia Cento, Lorenzo Nevi, Raffaella Mastrocola, Gustavo Ferreira Alves, Giuseppina Caretti, Massimo Collino, Fabio Penna
Journal of Advanced Research.2024;[Epub] CrossRef - Effects of Low-Load Blood Flow Restriction Training on Muscle Anabolism Biomarkers and Thrombotic Biomarkers Compared with Traditional Training in Healthy Adults Older Than 60 Years: Systematic Review and Meta-Analysis
Raúl Fabero-Garrido, Miguel Gragera-Vela, Tamara del Corral, Marta Hernández-Martín, Gustavo Plaza-Manzano, Ibai López-de-Uralde-Villanueva
Life.2024; 14(3): 411. CrossRef - Sarcopenia and non-alcoholic fatty liver disease
R. G. Myazin
Experimental and Clinical Gastroenterology.2024; (2): 120. CrossRef - THE ROLE OF DAIRY FOODS FOR HEALTHY AGING
Emine Kocyigit
Anti-Aging Eastern Europe.2024; 3(1): 23. CrossRef - Effects of Rosemary Extract on C2C12 Myoblast Differentiation and 5-Aminoimidazole-4-carboxamide Ribonucleoside (AICAR)-Induced Muscle Cell Atrophy
Jun Ho Lee, Jung Yoon Jang, Young Hoon Kwon, Seung Ho Lee, Cheol Park, Yung Hyun Choi, Nam Deuk Kim
Applied Sciences.2023; 13(2): 986. CrossRef - Relationship between asthma and sarcopenia in the elderly: a nationwide study from the KNHANES
Ha-Kyeong Won, Yewon Kang, Jin An, Ji-Hyang Lee, Woo-Jung Song, Hyouk-Soo Kwon, You Sook Cho, Hee-Bom Moon, Il-Young Jang, Tae-Bum Kim
Journal of Asthma.2023; 60(2): 304. CrossRef - A Lignan from Alnus japonica Activates Myogenesis and Alleviates Dexamethasone-induced Myotube Atrophy
Hyejin Lee, Ji Hye Jeong, Seung Hwan Hwang, Sung Hum Yeon, Jae-Ha Ryu
Planta Medica.2023; 89(05): 484. CrossRef - A cross-talk between sestrins, chronic inflammation and cellular senescence governs the development of age-associated sarcopenia and obesity
Gregory Livshits, Alexander Kalinkovich
Ageing Research Reviews.2023; 86: 101852. CrossRef - Facilitators and Barriers of Tai Chi Practice in Community-Dwelling Older Adults: Qualitative Study
Yan Du, Penny Roberts, Wei Liu
Asian/Pacific Island Nursing Journal.2023; 7: e42195. CrossRef - Extract of Alnus japonica prevents dexamethasone-induced muscle atrophy in mice
Hyejin Lee, Kyeong Seon Lee, Ji Hye Jeong, Ji Soo Yoon, Seung Hwan Hwang, Sang-Yoon Kim, Sung Hum Yeon, Jae-Ha Ryu
Journal of Functional Foods.2023; 101: 105419. CrossRef - Axis “microbiota - muscle”
A. N. Zavyalova, V. P. Novikova, P. D. Ignatova
Experimental and Clinical Gastroenterology.2023; (11): 60. CrossRef - Comparison of the effects of commercial whey protein and native whey protein on muscle strength and muscle protein synthesis in rats
Jiyun Kim, Eun Woo Jeong, Youjin Baek, Gwang-woong Go, Hyeon Gyu Lee
Food Science and Biotechnology.2023; 32(3): 381. CrossRef - Prevalence of sarcopenia in patients with COPD through different musculature measurements: An updated meta-analysis and meta-regression
Jie He, Hezhi Li, Jun Yao, Yan Wang
Frontiers in Nutrition.2023;[Epub] CrossRef - Proteome network analysis of skeletal muscle in lignan-enriched nutmeg extract-fed aged mice
Je-Ho Lee, Hyuno Kang, Gyung-Tae Ban, Beom Kyu Kim, JaeHyeon Lee, Heeyoun Hwang, Hwa-Seung Yoo, Kun Cho, Jong-Soon Choi
Journal of Analytical Science and Technology.2023;[Epub] CrossRef - Aging, Physical Exercise, Telomeres, and Sarcopenia: A Narrative Review
David Hernández-Álvarez, Juana Rosado-Pérez, Graciela Gavia-García, Taide Laurita Arista-Ugalde, Itzen Aguiñiga-Sánchez, Edelmiro Santiago-Osorio, Víctor Manuel Mendoza-Núñez
Biomedicines.2023; 11(2): 598. CrossRef - Genetic and Probiotic Characteristics of Urolithin A Producing Enterococcus faecium FUA027
Mengjie Xia, Shuting Mu, Yaowei Fang, Xiaomeng Zhang, Guang Yang, Xiaoyue Hou, Fuxiang He, Yaling Zhao, Yichen Huang, Wei Zhang, Juan Shen, Shu Liu
Foods.2023; 12(5): 1021. CrossRef - A nomogram to predict the risk of sarcopenia in older people
Guangjiao Yin, Juanjuan Qin, Ziwei Wang, Fang Lv, Xujun Ye
Medicine.2023; 102(16): e33581. CrossRef - Air pollution weaken your muscle? Evidence from a cross-sectional study on sarcopenia in central China
Faxue Zhang, Tianzhou Li, Bingbing Chen, Nuoya Li, Xupeng Zhang, Shijie Zhu, Gaichan Zhao, Xiaowei Zhang, TingTing Ma, Fang Zhou, Hao Liu, Wei Zhu
Ecotoxicology and Environmental Safety.2023; 258: 114962. CrossRef - Safety and Risk Factors of Needle Thoracentesis Decompression in Tension Pneumothorax in Patients over 75 Years Old
Yanhu Wang, Lei Wang, Cheng Chen, Yifan Que, Yinyi Li, Jiang Luo, Ming Yin, Miao Lv, Guogang Xu, Anita Pye
Canadian Respiratory Journal.2023; 2023: 1. CrossRef - Allosteric modulation of ryanodine receptor RyR1 by nucleotide derivatives
Spencer Cholak, James W. Saville, Xing Zhu, Alison M. Berezuk, Katharine S. Tuttle, Omid Haji-Ghassemi, Francisco J. Alvarado, Filip Van Petegem, Sriram Subramaniam
Structure.2023; 31(7): 790. CrossRef - Sarcopenia and Cardiovascular Diseases
Abdulla A. Damluji, Maha Alfaraidhy, Noora AlHajri, Namit N. Rohant, Manish Kumar, Christina Al Malouf, Samira Bahrainy, Min Ji Kwak, Wayne B. Batchelor, Daniel E. Forman, Michael W. Rich, James Kirkpatrick, Ashok Krishnaswami, Karen P. Alexander, Gary Ge
Circulation.2023; 147(20): 1534. CrossRef - Mitochondrial-Encoded Peptide MOTS-c, Diabetes, and Aging-Related Diseases
Byung Soo Kong, Changhan Lee, Young Min Cho
Diabetes & Metabolism Journal.2023; 47(3): 315. CrossRef - Pathogenesis, Intervention, and Current Status of Drug Development for Sarcopenia: A Review
Jung Yoon Jang, Donghwan Kim, Nam Deuk Kim
Biomedicines.2023; 11(6): 1635. CrossRef - Polyamines and Physical Activity in Musculoskeletal Diseases: A Potential Therapeutic Challenge
Letizia Galasso, Annalisa Cappella, Antonino Mulè, Lucia Castelli, Andrea Ciorciari, Alessandra Stacchiotti, Angela Montaruli
International Journal of Molecular Sciences.2023; 24(12): 9798. CrossRef - Non-alcoholic fatty liver disease in women – Current knowledge and emerging concepts
Pei Chia Eng, Roberta Forlano, Tricia Tan, Pinelopi Manousou, Waljit S. Dhillo, Chioma Izzi-Engbeaya
JHEP Reports.2023; 5(10): 100835. CrossRef - Effects of polyphenols and their metabolites on age-related diseases
Chouari Zhor, Lounis Wafaa, Imen Ghzaiel, Khadidja Kessas, Amira Zarrouk, Mohamed Ksila, Taoufik Ghrairi, Norbert Latruffe, Olfa Masmoudi-Kouki, Adil El Midaoui, Dominique Vervandier-Fasseur, Mohamed Hammami, Gérard Lizard, Anne Vejux, Omar Kharoubi
Biochemical Pharmacology.2023; 214: 115674. CrossRef - Circadian regulation in aging: Implications for spaceflight and life on earth
Deeksha Malhan, Britt Schoenrock, Müge Yalçin, Dieter Blottner, Angela Relόgio
Aging Cell.2023;[Epub] CrossRef - Mitochondria-associated programmed cell death as a therapeutic target for age-related disease
Thanh T. Nguyen, Shibo Wei, Thu Ha Nguyen, Yunju Jo, Yan Zhang, Wonyoung Park, Karim Gariani, Chang-Myung Oh, Hyeon Ho Kim, Ki-Tae Ha, Kyu Sang Park, Raekil Park, In-Kyu Lee, Minho Shong, Riekelt H. Houtkooper, Dongryeol Ryu
Experimental & Molecular Medicine.2023; 55(8): 1595. CrossRef - Normative reference values, determinants and regression equations for the incremental shuttle walk test (ISWT) in healthy Asian population aged 21 to 80 years
Muhammad Zulhaziq Bin Azman, Katherin S. Huang, Wei Jun Koh, Sarah S. Leong, Benjamin Ong, Johanna L. Soon, Sherman W. Tan, Melissa Y. Chan, Mingxing Yang, Meredith T. Yeung, Hans-Peter Kubis
PLOS ONE.2023; 18(9): e0291132. CrossRef - Influence of sarcopenia on postoperative complications in patients undergoing autologous microsurgical breast reconstruction: an inverse probability of treatment weighting analysis
Seung-Jun Lee, Yun-Jung Yang, Dong-Won Lee, Seung-Yong Song, Dae-Hyun Lew, Eun-Jung Yang
Frontiers in Oncology.2023;[Epub] CrossRef - Stromal vascular fraction in the treatment of myositis
S. Gandolfi, B. Pileyre, L. Drouot, I. Dubus, I. Auquit-Auckbur, J. Martinet
Cell Death Discovery.2023;[Epub] CrossRef - Multidisciplinary approach to sarcopenia: a narrative review
Wook Tae Park, Oog-Jin Shon, Gi Beom Kim
Journal of Yeungnam Medical Science.2023; 40(4): 352. CrossRef - Metallosis after Hip Arthroplasty Damages Skeletal Muscle: A Case Report
Roberto Bonanni, Lorenzo Abbondante, Ida Cariati, Elena Gasbarra, Umberto Tarantino
Geriatrics.2023; 8(5): 92. CrossRef - Hallmarks of ageing in human skeletal muscle and implications for understanding the pathophysiology of sarcopenia in women and men
Antoneta Granic, Karen Suetterlin, Tea Shavlakadze, Miranda D. Grounds, Avan A. Sayer
Clinical Science.2023; 137(22): 1721. CrossRef - Predicted lean body mass trajectories, and cancer risk and cancer‐specific and all‐cause mortality: A prospective cohort study
Chenan Liu, Qingsong Zhang, Tong Liu, Qi Zhang, Mengmeng Song, Guotian Ruan, Shiqi Lin, Ziwen Wang, Xin Zheng, Yue Chen, Heyang Zhang, Yizhong Ge, Hailun Xie, Jinyu Shi, Li Deng, Shouling Wu, Hanping Shi
Journal of Cachexia, Sarcopenia and Muscle.2023; 14(6): 2916. CrossRef - Cancer Cachexia: New Insights and Future Directions
Claudia Raluca Mariean, Oana Mirela Tiucă, Alexandru Mariean, Ovidiu Simion Cotoi
Cancers.2023; 15(23): 5590. CrossRef - Development and comparative analysis of protein-polyphenol-fibre bars as nutritional supplements suitable for healthy senior consumers
M. Jolji, B. Pecsenye, Z. Mposula, A. Aleya, T. Kiss, E. Mathé
Acta Universitatis Sapientiae, Alimentaria.2023; 16(1): 103. CrossRef - Zebrafish as an Emerging Model for Sarcopenia: Considerations, Current Insights, and Future Directions
Santiago Callegari, Foad Mirzaei, Lila Agbaria, Sanobar Shariff, Burhan Kantawala, Desmond Moronge, Brian M. O. Ogendi
International Journal of Molecular Sciences.2023; 24(23): 17018. CrossRef - Laboratory markers of osteosarcopenic obesity
O. V. Gritsenko, O. V. Gruzdeva, G. A. Chumakova, O. L. Barbarash
Russian Journal of Cardiology.2023; 28(12): 5563. CrossRef - Higher Plasma Stromal Cell-Derived Factor 1 Is Associated with Lower Risk for Sarcopenia in Older Asian Adults
Sunghwan Ji, Kyunggon Kim, So Jeong Park, Jin Young Lee, Hee-Won Jung, Hyun Ju Yoo, Il-Young Jang, Eunju Lee, Ji Yeon Baek, Beom-Jun Kim
Endocrinology and Metabolism.2023; 38(6): 701. CrossRef - Familial Correlation and Heritability of Hand Grip Strength in Korean Adults (Korea National Health and Nutrition Examination Survey 2014 to 2019)
Seong Hee Ahn, Eun Byeol Park, Seongha Seo, Yongin Cho, Da Hea Seo, So Hun Kim, Young Ju Suh, Seongbin Hong
Endocrinology and Metabolism.2023; 38(6): 709. CrossRef - Ageing with neuromuscular disease: Implications for a lifeworld‐led care through a humanising approach
Louise Abildgaard Møller, Bente Martinsen, Ulla Werlauf, Pia Dreyer
Journal of Clinical Nursing.2022; 31(17-18): 2507. CrossRef - CT-derived relationship between low relative muscle mass and bone damage in patients with multiple myeloma undergoing stem cells transplantation
Alberto Stefano Tagliafico, Federica Rossi, Bianca Bignotti, Lorenzo Torri, Alessandro Bonsignore, Liliana Belgioia, Alida Domineitto
The British Journal of Radiology.2022;[Epub] CrossRef - A new paradigm in sarcopenia: Cognitive impairment caused by imbalanced myokine secretion and vascular dysfunction
Danbi Jo, Gwangho Yoon, Oh Yoen Kim, Juhyun Song
Biomedicine & Pharmacotherapy.2022; 147: 112636. CrossRef - Sarcopenia Is a Cause and Consequence of Metabolic Dysregulation in Aging Humans: Effects of Gut Dysbiosis, Glucose Dysregulation, Diet and Lifestyle
James W. Daily, Sunmin Park
Cells.2022; 11(3): 338. CrossRef - Change of Computed Tomography-Based Body Composition after Adrenalectomy in Patients with Pheochromocytoma
Yousun Ko, Heeryoel Jeong, Seungwoo Khang, Jeongjin Lee, Kyung Won Kim, Beom-Jun Kim
Cancers.2022; 14(8): 1967. CrossRef - Acute bioenergetic insulin sensitivity of skeletal muscle cells: ATP-demand-provoked glycolysis contributes to stimulation of ATP supply
Rosie A. Donnell, Jane E. Carré, Charles Affourtit
Biochemistry and Biophysics Reports.2022; 30: 101274. CrossRef - The Hunt Is On! In Pursuit of the Ideal Stem Cell Population for Cartilage Regeneration
T. Mark Campbell, F. Jeffrey Dilworth, David S. Allan, Guy Trudel
Frontiers in Bioengineering and Biotechnology.2022;[Epub] CrossRef - Decreased Serum Level of Sclerostin in Older Adults with Sarcopenia
Seong Hee Ahn, Hee-Won Jung, Eunju Lee, Ji Yeon Baek, Il-Young Jang, So Jeong Park, Jin Young Lee, Eunah Choi, Yun Sun Lee, Seongbin Hong, Beom-Jun Kim
Endocrinology and Metabolism.2022; 37(3): 487. CrossRef - Effects of Muscles on Bone Metabolism—with a Focus on Myokines
Beom-Jun Kim
Annals of Geriatric Medicine and Research.2022; 26(2): 63. CrossRef - Sclerostin as a Putative Myokine in Sarcopenia
Hyon-Seung Yi
Endocrinology and Metabolism.2022; 37(3): 430. CrossRef - Slc12a8 in the lateral hypothalamus maintains energy metabolism and skeletal muscle functions during aging
Naoki Ito, Ai Takatsu, Hiromi Ito, Yuka Koike, Kiyoshi Yoshioka, Yasutomi Kamei, Shin-ichiro Imai
Cell Reports.2022; 40(4): 111131. CrossRef - Restoration of NAD+homeostasis protects C2C12 myoblasts and mouse levator ani muscle from mechanical stress-induced damage
Guotao Huang, Yong He, Li Hong, Min Zhou, Xiaohu Zuo, Zhihan Zhao
Animal Cells and Systems.2022; 26(4): 192. CrossRef - Machine learning-featured Secretogranin V is a circulating diagnostic biomarker for pancreatic adenocarcinomas associated with adipopenia
Yunju Jo, Min-Kyung Yeo, Tam Dao, Jeongho Kwon, Hyon‐Seung Yi, Dongryeol Ryu
Frontiers in Oncology.2022;[Epub] CrossRef - Development and Verification of a Combined Diagnostic Model for Sarcopenia with Random Forest and Artificial Neural Network
Shangjin Lin, Cong Chen, Xiaoxi Cai, Fengjian Yang, Yongqian Fan, Rajesh Kaluri
Computational and Mathematical Methods in Medicine.2022; 2022: 1. CrossRef - Bronchial Asthma and Sarcopenia: An Upcoming Potential Interaction
Nikolaos D. Karakousis, Ourania S. Kotsiou, Konstantinos I. Gourgoulianis
Journal of Personalized Medicine.2022; 12(10): 1556. CrossRef - Suppressive Effects of Turmeric Extract on Muscle Atrophy in Dexamethasone-Treated Mice and Myotubes
Kyohei Furukawa, Marika Kousaka, Huijuan Jia, Hisanori Kato
Nutrients.2022; 14(19): 3979. CrossRef - Normative reference values and regression equations to predict the 6-minute walk distance in the Asian adult population aged 21–80 years
Meredith T. Yeung, Melissa Y. Chan, Katherin S. Huang, Tian Jie Chen, Cyprian P. Chia, Meihiko M. Fong, Cherilyn S. Ho, Derek T. Koh, Mitchell J. Neo, Mark Tan
Hong Kong Physiotherapy Journal.2022; 42(02): 111. CrossRef - Food insecurity as a risk factor of sarcopenic obesity in older adults
Diana Fonseca-Pérez, Cecilia Arteaga-Pazmiño, Claudia P. Maza-Moscoso, Sara Flores-Madrid, Ludwig Álvarez-Córdova
Frontiers in Nutrition.2022;[Epub] CrossRef - Transcriptome Sequencing Analysis of circRNA in Skeletal Muscle between Fast- and Slow-Growing Chickens at Embryonic Stages
Genxi Zhang, Jin Zhang, Pengfei Wu, Xuanze Ling, Qifan Wang, Kaizhi Zhou, Peifeng Li, Li Zhang, Hongxin Ye, Qi Zhang, Qingyu Wei, Tao Zhang, Xinglong Wang
Animals.2022; 12(22): 3166. CrossRef - Amino Acids in Cancer and Cachexia: An Integrated View
Maurizio Ragni, Claudia Fornelli, Enzo Nisoli, Fabio Penna
Cancers.2022; 14(22): 5691. CrossRef - Determination of skeletal muscle mass by aspartate aminotransferase / alanine aminotransferase ratio, insulin and FSH in Chinese women with sarcopenia
Mengting Yin, He Zhang, Qianhui Liu, Fei Ding, Lisha Hou, Yiping Deng, Tao Cui, Yixian Han, Yijun Chen, Chen Huang, Jirong Yue, Yong He
BMC Geriatrics.2022;[Epub] CrossRef - The inflammatory response, a mixed blessing for muscle homeostasis and plasticity
Zineb Bouredji, Anteneh Argaw, Jérôme Frenette
Frontiers in Physiology.2022;[Epub] CrossRef - Effect of CCL11 on In Vitro Myogenesis and Its Clinical Relevance for Sarcopenia in Older Adults
Da Ae Kim, So Jeong Park, Jin Young Lee, Jeoung Hee Kim, Seungjoo Lee, Eunju Lee, Il-Young Jang, Hee-Won Jung, Jin Hoon Park, Beom-Jun Kim
Endocrinology and Metabolism.2021; 36(2): 455. CrossRef - Association of Water Intake with Hand Grip Strength in Community-Dwelling Older Adults
Hyeonmok Kim, Sun Hee Beom, Tae Ho Kim, Beom-Jun Kim
Nutrients.2021; 13(6): 1756. CrossRef - Receptor-Mediated Muscle Homeostasis as a Target for Sarcopenia Therapeutics
Jong Hyeon Yoon, Ki-Sun Kwon
Endocrinology and Metabolism.2021; 36(3): 478. CrossRef - Redox Signaling and Sarcopenia: Searching for the Primary Suspect
Nicholas A. Foreman, Anton S. Hesse, Li Li Ji
International Journal of Molecular Sciences.2021; 22(16): 9045. CrossRef - Aldosterone Inhibits In Vitro Myogenesis by Increasing Intracellular Oxidative Stress via Mineralocorticoid Receptor
Jin Young Lee, Da Ae Kim, Eunah Choi, Yun Sun Lee, So Jeong Park, Beom-Jun Kim
Endocrinology and Metabolism.2021; 36(4): 865. CrossRef - Artificial‐intelligence‐driven discovery of prognostic biomarker for sarcopenia
Heewon Chung, Yunju Jo, Dongryeol Ryu, Changwon Jeong, Seong‐Kyu Choe, Jinseok Lee
Journal of Cachexia, Sarcopenia and Muscle.2021; 12(6): 2220. CrossRef - Association between Neurologic Outcomes and Changes of Muscle Mass Measured by Brain Computed Tomography in Neurocritically Ill Patients
Yun Im Lee, Ryoung-Eun Ko, Joonghyun Ahn, Keumhee C. Carriere, Jeong-Am Ryu
Journal of Clinical Medicine.2021; 11(1): 90. CrossRef - Computed Tomography-Derived Skeletal Muscle Radiodensity Is an Early, Sensitive Marker of Age-Related Musculoskeletal Changes in Healthy Adults
Yeon Woo Jung, Namki Hong, Joon Chae Na, Woong Kyu Han, Yumie Rhee
Endocrinology and Metabolism.2021; 36(6): 1201. CrossRef
- Interactions between mitochondrial dysfunction and other hallmarks of aging: Paving a path toward interventions that promote healthy old age

- Obesity and Metabolism
- Implications of Mitochondrial Unfolded Protein Response and Mitokines: A Perspective on Fatty Liver Diseases
- Hyon-Seung Yi
- Endocrinol Metab. 2019;34(1):39-46. Published online March 21, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.1.39
- 5,862 View
- 140 Download
- 25 Web of Science
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub The signaling network of the mitochondrial unfolded protein response (UPRmt) and mitohormesis is a retrograde signaling pathway through which mitochondria-to-nucleus communication occurs in organisms. Recently, it has been shown that the UPRmt is closely associated with metabolic disorders and conditions involving insulin resistance, such as alcoholic and non-alcoholic fatty liver and fibrotic liver disease. Scientific efforts to understand the UPRmt and mitohormesis, as well as to establish the mitochondrial proteome, have established the importance of mitochondrial quality control in the development and progression of metabolic liver diseases, including non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). In this review, we integrate and discuss the recent data from the literature on the UPRmt and mitohormesis in metabolic liver diseases, including NAFLD/NASH and fibrosis.
-
Citations
Citations to this article as recorded by- Aberrant mitochondrial aggregation of TDP-43 activated mitochondrial unfolded protein response and contributed to recovery of acetaminophen induced acute liver injury
Zhaoxiong Liu, Yalong Qiang, Shulin Shan, Shuai Wang, Zhidan Liu, Yiyu Yang, Zhengcheng Huang, Mingxue Song, Xiulan Zhao, Fuyong Song
Toxicology Research.2024;[Epub] CrossRef - Autophagy and the unfolded protein response shape the non-alcoholic fatty liver landscape: decoding the labyrinth
Zahra Dashti, Zeynab Yousefi, Pouria Kiani, Motahareh Taghizadeh, Mohammad Hasan Maleki, Mohammad Borji, Omid Vakili, Sayed Mohammad Shafiee
Metabolism.2024; 154: 155811. CrossRef - Recent progress in understanding mitokines as diagnostic and therapeutic targets in hepatocellular carcinoma
Jiang Wang, Lan-Zhu Luo, Dao-Miao Liang, Chao Guo, Zhi-Hong Huang, Xiao-Hong Jian, Jie Wen
World Journal of Clinical Cases.2023; 11(23): 5416. CrossRef - From Non-Alcoholic Fatty Liver to Hepatocellular Carcinoma: A Story of (Mal)Adapted Mitochondria
Ricardo Amorim, Carina C. Magalhães, Fernanda Borges, Paulo J. Oliveira, José Teixeira
Biology.2023; 12(4): 595. CrossRef - Natural Drugs: A New Direction for the Prevention and Treatment of Diabetes
Peishan Wu, Xiaolei Wang
Molecules.2023; 28(14): 5525. CrossRef - Mitochondrial Quality Control via Mitochondrial Unfolded Protein Response (mtUPR) in Ageing and Neurodegenerative Diseases
Paula Cilleros-Holgado, David Gómez-Fernández, Rocío Piñero-Pérez, Jose Manuel Romero-Domínguez, Diana Reche-López, Alejandra López-Cabrera, Mónica Álvarez-Córdoba, Manuel Munuera-Cabeza, Marta Talaverón-Rey, Alejandra Suárez-Carrillo, Ana Romero-González
Biomolecules.2023; 13(12): 1789. CrossRef - Heat Shock Protein 60 Restricts Release of Mitochondrial dsRNA to Suppress Hepatic Inflammation and Ameliorate Non-Alcoholic Fatty Liver Disease in Mice
Ying-Hsien Huang, Feng-Sheng Wang, Pei-Wen Wang, Hung-Yu Lin, Sheng-Dean Luo, Ya-Ling Yang
International Journal of Molecular Sciences.2022; 23(1): 577. CrossRef - UPRmt activation improves pathological alterations in cellular models of mitochondrial diseases
Juan M. Suárez-Rivero, Carmen J. Pastor-Maldonado, Suleva Povea-Cabello, Mónica Álvarez-Córdoba, Irene Villalón-García, Marta Talaverón-Rey, Alejandra Suárez-Carrillo, Manuel Munuera-Cabeza, Diana Reche-López, Paula Cilleros-Holgado, Rocío Piñero-Perez, J
Orphanet Journal of Rare Diseases.2022;[Epub] CrossRef - Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell
Kyung-Soo Kim, Yeon Kyung Choi, Mi Jin Kim, Jung Wook Hwang, Kyunghoon Min, Sang Youn Jung, Soo-Kyung Kim, Yong-Soo Choi, Yong-Wook Cho
Diabetes & Metabolism Journal.2021; 45(2): 260. CrossRef - Mitochondrial unfolded protein response: An emerging pathway in human diseases
Li Zhu, Qionglin Zhou, Lu He, Linxi Chen
Free Radical Biology and Medicine.2021; 163: 125. CrossRef - Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): new perspectives for a fairy-tale ending?
Miriam Longo, Marica Meroni, Erika Paolini, Chiara Macchi, Paola Dongiovanni
Metabolism.2021; 117: 154708. CrossRef - Mitochondrial unfolded protein response: A novel pathway in metabolism and immunity
Li Zhu, Xuling Luo, Nian Fu, Linxi Chen
Pharmacological Research.2021; 168: 105603. CrossRef - Mitochondrial Metabolic Signatures in Hepatocellular Carcinoma
Ho-Yeop Lee, Ha Thi Nga, Jingwen Tian, Hyon-Seung Yi
Cells.2021; 10(8): 1901. CrossRef - Cnm1 mediates nucleus–mitochondria contact site formation in response to phospholipid levels
Michal Eisenberg-Bord, Naama Zung, Javier Collado, Layla Drwesh, Emma J. Fenech, Amir Fadel, Nili Dezorella, Yury S. Bykov, Doron Rapaport, Ruben Fernandez-Busnadiego, Maya Schuldiner
Journal of Cell Biology.2021;[Epub] CrossRef - The Role of Carnitine Orotate Complex in Fatty Liver
Hyon-Seung Yi
Diabetes & Metabolism Journal.2021; 45(6): 866. CrossRef - Serum GDF15 Level Is Independent of Sarcopenia in Older Asian Adults
Ha Thi Nga, Il-Young Jang, Da Ae Kim, So Jeong Park, Jin Young Lee, Seungjoo Lee, Jeoung Hee Kim, Eunju Lee, Jin Hoon Park, Young-Ho Lee, Hyon-Seung Yi, Beom-Jun Kim
Gerontology.2021; 67(5): 525. CrossRef - Exogenous Therapeutics of Microrna-29a Attenuates Development of Hepatic Fibrosis in Cholestatic Animal Model through Regulation of Phosphoinositide 3-Kinase p85 Alpha
Ya-Ling Yang, Feng-Sheng Wang, Hung-Yu Lin, Ying-Hsien Huang
International Journal of Molecular Sciences.2020; 21(10): 3636. CrossRef - High-altitude chronic hypoxia ameliorates obesity-induced non-alcoholic fatty liver disease in mice by regulating mitochondrial and AMPK signaling
Kang Song, Yifan Zhang, Qin Ga, Zhenzhong Bai, Ri-Li Ge
Life Sciences.2020; 252: 117633. CrossRef - NRF-2 and nonalcoholic fatty liver disease
Arturo Solano-Urrusquieta, José A. Morales-González, Graciela E. Castro-Narro, Eira Cerda-Reyes, Perla D. Flores-Rangel, Raul Fierros-Oceguera
Annals of Hepatology.2020; 19(5): 458. CrossRef - Mitochondrial Quality Control in the Heart: New Drug Targets for Cardiovascular Disease
Chang-Myung Oh, Dongryeol Ryu, Sungsoo Cho, Yangsoo Jang
Korean Circulation Journal.2020; 50(5): 395. CrossRef - Growth differentiation factor 15 protects against the aging‐mediated systemic inflammatory response in humans and mice
Ji Sun Moon, Ludger J. E. Goeminne, Jung Tae Kim, Jing Wen Tian, Seok‐Hwan Kim, Ha Thi Nga, Seul Gi Kang, Baeki E. Kang, Jin‐Seok Byun, Young‐Sun Lee, Jae‐Han Jeon, Minho Shong, Johan Auwerx, Dongryeol Ryu, Hyon‐Seung Yi
Aging Cell.2020;[Epub] CrossRef - miR-29a Modulates GSK3β/SIRT1-Linked Mitochondrial Proteostatic Stress to Ameliorate Mouse Non-Alcoholic Steatohepatitis
Ya-Ling Yang, Pei-Wen Wang, Feng-Sheng Wang, Hung-Yu Lin, Ying-Hsien Huang
International Journal of Molecular Sciences.2020; 21(18): 6884. CrossRef - Impact of Mitophagy and Mitochondrial Unfolded Protein Response as New Adaptive Mechanisms Underlying Old Pathologies: Sarcopenia and Non-Alcoholic Fatty Liver Disease
Rodrigo Urbina-Varela, Nataly Castillo, Luis A. Videla, Andrea del Campo
International Journal of Molecular Sciences.2020; 21(20): 7704. CrossRef - Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease
Jin Lee, Jeong-Su Park, Yoon Seok Roh
Archives of Pharmacal Research.2019; 42(11): 935. CrossRef - A siRNA mediated hepatic dpp4 knockdown affects lipid, but not glucose metabolism in diabetic mice
Sven Wolfgang Görgens, Kerstin Jahn-Hofmann, Dinesh Bangari, Sheila Cummings, Christiane Metz-Weidmann, Uwe Schwahn, Paulus Wohlfart, Matthias Schäfer, Maximilian Bielohuby, Marcia B. Aguila
PLOS ONE.2019; 14(12): e0225835. CrossRef
- Aberrant mitochondrial aggregation of TDP-43 activated mitochondrial unfolded protein response and contributed to recovery of acetaminophen induced acute liver injury

- Endocrine Research
- Deficiency of Sphingosine-1-Phosphate Reduces the Expression of Prohibitin and Causes β-Cell Impairment via Mitochondrial Dysregulation
- Seok-Woo Hong, Jinmi Lee, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Cheol-Young Park, Ki-Won Oh, Sung-Woo Park, Won-Young Lee
- Endocrinol Metab. 2018;33(3):403-412. Published online September 18, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.3.403
- 4,192 View
- 50 Download
- 16 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Emerging evidence suggests that sphingolipids may be involved in type 2 diabetes. However, the exact signaling defect through which disordered sphingolipid metabolism induces β-cell dysfunction remains unknown. The current study demonstrated that sphingosine-1-phosphate (S1P), the product of sphingosine kinase (SphK), is an essential factor for maintaining β-cell function and survival via regulation of mitochondrial action, as mediated by prohibitin (PHB).
Methods We examined β-cell function and viability, as measured by mitochondrial function, in mouse insulinoma 6 (MIN6) cells in response to manipulation of cellular S1P and PHB levels.
Results Lack of S1P induced by sphingosine kinase inhibitor (SphKi) treatment caused β-cell dysfunction and apoptosis, with repression of mitochondrial function shown by decreases in cellular adenosine triphosphate content, the oxygen consumption rate, the expression of oxidative phosphorylation complexes, the mitochondrial membrane potential, and the expression of key regulators of mitochondrial dynamics (mitochondrial dynamin-like GTPase [OPA1] and mitofusin 1 [MFN1]). Supplementation of S1P led to the recovery of mitochondrial function and greatly improved β-cell function and viability. Knockdown of SphK2 using small interfering RNA induced mitochondrial dysfunction, decreased glucose-stimulated insulin secretion (GSIS), and reduced the expression of PHB, an essential regulator of mitochondrial metabolism. PHB deficiency significantly reduced GSIS and induced mitochondrial dysfunction, and co-treatment with S1P did not reverse these trends.
Conclusion Altogether, these data suggest that S1P is an essential factor in the maintenance of β-cell function and survival through its regulation of mitochondrial action and PHB expression.
-
Citations
Citations to this article as recorded by- Mitochondrial Cristae Morphology Reflecting Metabolism, Superoxide Formation, Redox Homeostasis, and Pathology
Petr Ježek, Martin Jabůrek, Blanka Holendová, Hana Engstová, Andrea Dlasková
Antioxidants & Redox Signaling.2023; 39(10-12): 635. CrossRef - Sphingolipids in mitochondria—from function to disease
Maryam Jamil, Lauren Ashley Cowart
Frontiers in Cell and Developmental Biology.2023;[Epub] CrossRef - Sphingosine‐1‐phosphate in mitochondrial function and metabolic diseases
Meng Duan, Pan Gao, Sheng‐xi Chen, Petr Novák, Kai Yin, Xiao Zhu
Obesity Reviews.2022;[Epub] CrossRef - Involvement of miR‐27a‐3p in diabetic nephropathy via affecting renal fibrosis, mitochondrial dysfunction, and endoplasmic reticulum stress
Lina Wu, Qingzhu Wang, Feng Guo, Xiaojun Ma, Jiao Wang, Yanyan Zhao, Yushan Yan, Guijun Qin
Journal of Cellular Physiology.2021; 236(2): 1454. CrossRef - Sphingosine‐1‐phosphate in acute exercise and training
Katarzyna Hodun, Adrian Chabowski, Marcin Baranowski
Scandinavian Journal of Medicine & Science in Sports.2021; 31(5): 945. CrossRef - The Ethyl Acetate Extract From Celastrus orbiculatus Promotes Apoptosis of Gastric Cancer Cells Through Mitochondria Regulation by PHB
Lide Tao, Zixin Yin, Tengyang Ni, Zewen Chu, Shihua Hao, Zeyu Wang, Masataka Sunagawa, Haibo Wang, Yanqing Liu
Frontiers in Pharmacology.2021;[Epub] CrossRef - Sphingosine 1-phosphate Stimulates Insulin Secretion and Improves Cell Survival by Blocking Voltage-dependent K+ Channels in β Cells
Zhihong Liu, Huanhuan Yang, Linping Zhi, Huan Xue, Zhihong Lu, Yanli Zhao, Lijuan Cui, Tao Liu, Shouan Ren, Peifeng He, Yunfeng Liu, Yi Zhang
Frontiers in Pharmacology.2021;[Epub] CrossRef - Sphingosine-1 Phosphate Lyase Regulates Sensitivity of Pancreatic Beta-Cells to Lipotoxicity
Yadi Tang, Thomas Plötz, Markus H. Gräler, Ewa Gurgul-Convey
International Journal of Molecular Sciences.2021; 22(19): 10893. CrossRef - Sphingolipids and Mitochondrial Dynamic
Lais Brigliadori Fugio, Fernanda B. Coeli-Lacchini, Andréia Machado Leopoldino
Cells.2020; 9(3): 581. CrossRef - Diminished Sphingolipid Metabolism, a Hallmark of Future Type 2 Diabetes Pathogenesis, Is Linked to Pancreatic β Cell Dysfunction
Saifur R. Khan, Yousef Manialawy, Andreea Obersterescu, Brian J. Cox, Erica P. Gunderson, Michael B. Wheeler
iScience.2020; 23(10): 101566. CrossRef - Neuronal Metabolism and Neuroprotection: Neuroprotective Effect of Fingolimod on Menadione-Induced Mitochondrial Damage
Antonio Gil, Elisa Martín-Montañez, Nadia Valverde, Estrella Lara, Federica Boraldi, Silvia Claros, Silvana-Yanina Romero-Zerbo, Oscar Fernández, Jose Pavia, Maria Garcia-Fernandez
Cells.2020; 10(1): 34. CrossRef - WITHDRAWN: Ceramide and Sphingosine 1-Phosphate in adipose dysfunction
Zijian Fang, Susan Pyne, Nigel J. Pyne
Progress in Lipid Research.2019; : 100991. CrossRef - Dynamic of mitochondrial network, cristae, and mitochondrial nucleoids in pancreatic β-cells
Petr Ježek, Andrea Dlasková
Mitochondrion.2019; 49: 245. CrossRef - Sphingosine kinase 1 overexpression induces MFN2 fragmentation and alters mitochondrial matrix Ca2+ handling in HeLa cells
I. Pulli, C. Löf, T. Blom, M.Y. Asghar, T. Lassila, N. Bäck, K.-L. Lin, J.H. Nyström, K. Kemppainen, D.M. Toivola, E. Dufour, A. Sanz, H.M. Cooper, J.B. Parys, K. Törnquist
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research.2019; 1866(9): 1475. CrossRef - Ceramide and sphingosine 1-phosphate in adipose dysfunction
Zijian Fang, Susan Pyne, Nigel J. Pyne
Progress in Lipid Research.2019; 74: 145. CrossRef - S1P/S1P Receptor Signaling in Neuromuscolar Disorders
Elisabetta Meacci, Mercedes Garcia-Gil
International Journal of Molecular Sciences.2019; 20(24): 6364. CrossRef
- Mitochondrial Cristae Morphology Reflecting Metabolism, Superoxide Formation, Redox Homeostasis, and Pathology

- Diabetes-Related Cardiac Dysfunction
- Lamario J. Williams, Brenna G. Nye, Adam R. Wende
- Endocrinol Metab. 2017;32(2):171-179. Published online June 23, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.2.171
- 12,180 View
- 45 Download
- 37 Web of Science
- 36 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub The proposal that diabetes plays a role in the development of heart failure is supported by the increased risk associated with this disease, even after correcting for all other known risk factors. However, the precise mechanisms contributing to the condition referred to as diabetic cardiomyopathy have remained elusive, as does defining the disease itself. Decades of study have defined numerous potential factors that each contribute to disease susceptibility, progression, and severity. Many recent detailed reviews have been published on mechanisms involving insulin resistance, dysregulation of microRNAs, and increased reactive oxygen species, as well as causes including both modifiable and non-modifiable risk factors. As such, the focus of the current review is to highlight aspects of each of these topics and to provide specific examples of recent advances in each area.
-
Citations
Citations to this article as recorded by-
Upregulation of PCSK9, rho kinase and cardiac troponin by
Eucalyptus globulus
leaf extract improves fructose-streptozotocin-induced diabetic cardiac dysfunction in rats
Afolabi C. Akinmoladun, Morenikejimi Bello, Emmanuel Oluwafemi Ibukun
Archives of Physiology and Biochemistry.2023; 129(6): 1219. CrossRef - An Overview of Cardiotonic Medicinal Plants from the Perspective of Iranian Traditional Medicine
Akram Alembagheri, Homa Hajimehdipoor, Rasool Choopani, Somayeh Esmaeili
Jundishapur Journal of Natural Pharmaceutical Products.2023;[Epub] CrossRef - Nanoformulations for the Delivery of Dietary Anthocyanins for the Prevention and Treatment of Diabetes Mellitus and Its Complications
Ana R. Nunes, Elisabete C. Costa, Gilberto Alves, Luís R. Silva
Pharmaceuticals.2023; 16(5): 736. CrossRef - Cyp2e1 knockdown attenuates high glucose-induced apoptosis and oxidative stress of cardiomyocytes by activating PI3K/Akt signaling
Jianying Wang, Han Yang, Chao Wang, Cuie Kan
Acta Diabetologica.2023; 60(9): 1219. CrossRef - Role of vascular endothelial growth factor B in nonalcoholic fatty liver disease and its potential value
Yu-Qi Li, Lei Xin, Yu-Chi Zhao, Shang-Qi Li, Ya-Nuo Li
World Journal of Hepatology.2023; 15(6): 786. CrossRef - Non-Invasive Assessment of the Intraventricular Pressure Using Novel Color M-Mode Echocardiography in Animal Studies: Current Status and Future Perspectives in Veterinary Medicine
Ahmed S. Mandour, Ahmed Farag, Mahmoud A. Y. Helal, Gamal El-Masry, Salim Al-Rejaie, Ken Takahashi, Tomohiko Yoshida, Lina Hamabe, Ryou Tanaka
Animals.2023; 13(15): 2452. CrossRef - Diet‐induced prediabetes: Effects on the activity of the renin–angiotensin–aldosterone system in selected organs
Bongeka Cassandra Mkhize, Palesa Mosili, Phikelelani Sethu Ngubane, Ntethelelo Hopewell Sibiya, Andile Khathi
Journal of Diabetes Investigation.2022; 13(5): 768. CrossRef - Knowledge domain and emerging trends in diabetic cardiomyopathy: A scientometric review based on CiteSpace analysis
Shiyi Tao, Deshuang Yang, Lanxin Zhang, Lintong Yu, Zihan Wang, Lingling Li, Jin Zhang, Ruiqi Yao, Li Huang, Mingjing Shao
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Clinical Evidence and Proposed Mechanisms for Cardiovascular and Kidney Benefits from Sodium–Glucose Co-transporter-2 Inhibitors
Joshua J Neumiller, Fredrick J Lienhard, Radica Z Alicic, Katherine R Tuttle
European Endocrinology.2022; 18(2): 106. CrossRef - Protective effects of medicinal plant against diabetes induced cardiac disorder: A review
Sadegh Shabab, Zahra Gholamnezhad, Maryam Mahmoudabady
Journal of Ethnopharmacology.2021; 265: 113328. CrossRef - Toward a broader view of mechanisms of drug cardiotoxicity
Polina Mamoshina, Blanca Rodriguez, Alfonso Bueno-Orovio
Cell Reports Medicine.2021; 2(3): 100216. CrossRef - Cardioprotective Effect of Glycyrrhizin on Myocardial Remodeling in Diabetic Rats
Vikram Thakur, Narah Alcoreza, Monica Delgado, Binata Joddar, Munmun Chattopadhyay
Biomolecules.2021; 11(4): 569. CrossRef - Cardioprotective Action of Glycyrrhizin on Diabetic Rats with Myocardial Remodeling
Fuxu Chen, Jie Song, Enas Abdulhay
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef - Management of inflammation in cardiovascular diseases
Sumanta Kumar Goswami, Prabhat Ranjan, Roshan Kumar Dutta, Suresh Kumar Verma
Pharmacological Research.2021; 173: 105912. CrossRef - Diabetic Cardiomyopathy: Clinical and Metabolic Approach
Dragan B. Djordjevic, Goran Koracevic, Aleksandar D. Djordjevic, Dragan B. Lovic
Current Vascular Pharmacology.2021; 19(5): 487. CrossRef - Effect of Acute Chemotherapy on Glucose Levels in Rats
Ahmad H. Alhowail, Gena S. Alfawzan, Maha A. Aldubayan, Lolwah S. Alsalam
International Journal of Pharmacology.2020; 16(3): 276. CrossRef - Transplantation of adipose tissue lacking PAI-1 improves glucose tolerance and attenuates cardiac metabolic abnormalities in high-fat diet-induced obesity
Sijing Liu, Yi Li, Xin Fan, Kai Li, Chunrong Xu, Liping Zhang, Mao Luo, Liqun Wang, Rong Li, Jianbo Wu
Adipocyte.2020; 9(1): 170. CrossRef - Cardiometabolic-Based Chronic Disease, Adiposity and Dysglycemia Drivers
Jeffrey I. Mechanick, Michael E. Farkouh, Jonathan D. Newman, W. Timothy Garvey
Journal of the American College of Cardiology.2020; 75(5): 525. CrossRef - Associated Targets of the Antioxidant Cardioprotection of Ganoderma lucidum in Diabetic Cardiomyopathy by Using Open Targets Platform: A Systematic Review
Fahmi Shaher, Hongbin Qiu, Shuqiu Wang, Yu Hu, Weiqun Wang, Yu Zhang, Yao Wei, Hisham AL-ward, Mahfoudh A. M. Abdulghani, Sattam Khulaif Alenezi, Salem Baldi, Shaobo Zhou
BioMed Research International.2020; 2020: 1. CrossRef - Human trophoblast-derived exosomes attenuate doxorubicin-induced cardiac injury by regulating miR-200b and downstream Zeb1
Jie Ni, Yihai Liu, Lina Kang, Lian Wang, Zhonglin Han, Kun Wang, Biao Xu, Rong Gu
Journal of Nanobiotechnology.2020;[Epub] CrossRef - Clinical Evidence and Proposed Mechanisms for Cardiovascular and Kidney Benefits from Glucagon-like Peptide-1 Receptor Agonists
Emily J Cox, Radica Z Alicic, Joshua J Neumiller, Katherine R Tuttle
US Endocrinology.2020; 16(2): 80. CrossRef - Hyperbaric Oxygen Therapy Dampens Inflammatory Cytokine Production and Does Not Worsen the Cardiac Function and Oxidative State of Diabetic Rats
Rita Benkő, Zsuzsanna Miklós, Viktor Antal Ágoston, Katrine Ihonvien, Csaba Répás, Roland Csépányi-Kömi, Margit Kerék, Nóra Judit Béres, Eszter Mária Horváth
Antioxidants.2019; 8(12): 607. CrossRef - Heart Failure in Type 2 Diabetes Mellitus
Helena C. Kenny, E. Dale Abel
Circulation Research.2019; 124(1): 121. CrossRef - SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob−/− mice
Damilola D. Adingupu, Sven O. Göpel, Julia Grönros, Margareta Behrendt, Matus Sotak, Tasso Miliotis, Ulrika Dahlqvist, Li-Ming Gan, Ann-Cathrine Jönsson-Rylander
Cardiovascular Diabetology.2019;[Epub] CrossRef - Depressive symptoms in asymptomatic stage B heart failure with Type II diabetic mellitus
Paul J. Mills, Pam R. Taub, Ottar Lunde, Meredith A. Pung, Kathleen Wilson, Christopher Pruitt, Thomas Rutledge, Alan Maisel, Barry H. Greenberg
Clinical Cardiology.2019; 42(6): 637. CrossRef - Pathophysiological mechanisms of diabetic cardiomyopathy and the therapeutic potential of epigallocatechin-3-gallate
Amir M. Al Hroob, Mohammad H. Abukhalil, Omnia E. Hussein, Ayman M. Mahmoud
Biomedicine & Pharmacotherapy.2019; 109: 2155. CrossRef - The Janus face of HMGB1 in heart disease: a necessary update
Angela Raucci, Stefania Di Maggio, Francesco Scavello, Alessandro D’Ambrosio, Marco E. Bianchi, Maurizio C. Capogrossi
Cellular and Molecular Life Sciences.2019; 76(2): 211. CrossRef - Histological evidence of chitosan-encapsulated curcumin suppresses heart and kidney damages on streptozotocin-induced type-1 diabetes in mice model
Sabri Sudirman, Ching-Shu Lai, Yi-Ling Yan, Hung-I Yeh, Zwe-Ling Kong
Scientific Reports.2019;[Epub] CrossRef - Microarray profiling analysis identifies the mechanism of miR‐200b‐3p/mRNA‐CD36 affecting diabetic cardiomyopathy via peroxisome proliferator activated receptor‐γ signaling pathway
Liqiong Xu, Wei Chen, Min Ma, Anfang Chen, Chengyue Tang, Chengwei Zhang, Lin Cai
Journal of Cellular Biochemistry.2019; 120(4): 5193. CrossRef - Plasma Low-Density Lipoprotein Cholesterol Correlates With Heart Function in Individuals With Type 2 Diabetes Mellitus: A Cross-Sectional Study
Po-Chung Cheng, Shang-Ren Hsu, Jung-Chi Li, Ching-Pei Chen, Szu-Chi Chien, Shih-Te Tu, Yun-Chung Cheng, Yu-Hsiu Liu, Jeng-Fu Kuo
Frontiers in Endocrinology.2019;[Epub] CrossRef - Impact of diabetes mellitus on the contractile properties of the left and right atrial myofilaments†
Constanze Bening, Khaled Alhussini, Elena-Aura Mazalu, Jonathan Yaqub, Khaled Hamouda, Dejan Radakovic, Christoph Schimmer, Grzegorz Hirnle, Rainer Leyh
European Journal of Cardio-Thoracic Surgery.2018; 54(5): 826. CrossRef - LAZ3 protects cardiac remodeling in diabetic cardiomyopathy via regulating miR-21/PPARa signaling
Lu Gao, Yuan Liu, Sen Guo, Lili Xiao, Leiming Wu, Zheng Wang, Cui Liang, Rui Yao, Yanzhou Zhang
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2018; 1864(10): 3322. CrossRef - Gene expression profiles of rat MMECs with different glucose levels and fgl2 gene silencing
Zhenzhong Zheng, Fan Zhang, Dengpeng Gao, Yujing Wu, Hao Wu
Diabetes/Metabolism Research and Reviews.2018;[Epub] CrossRef - Empagliflozin Ammeliorates High Glucose Induced-Cardiac Dysfuntion in Human iPSC-Derived Cardiomyocytes
Kwong-Man Ng, Yee-Man Lau, Vidhu Dhandhania, Zhu-Jun Cai, Yee-Ki Lee, Wing-Hon Lai, Hung-Fat Tse, Chung-Wah Siu
Scientific Reports.2018;[Epub] CrossRef - Apelin‑13 ameliorates metabolic and cardiovascular disorders in a rat model of type 2 diabetes with a high‑fat diet
Meng Li, Huijuan Fang, Jian Hu
Molecular Medicine Reports.2018;[Epub] CrossRef - Adriamycin-induced cardiomyopathy can serve as a model for diabetic cardiomyopathy – a hypothesis
Kaviyarasi Renu, V.G. Abilash, P.B. Tirupathi Pichiah, Thabassum Akthar Syeda, Sankarganesh Arunachalam
Asian Pacific Journal of Tropical Biomedicine.2017; 7(11): 1041. CrossRef
-
Upregulation of PCSK9, rho kinase and cardiac troponin by
Eucalyptus globulus
leaf extract improves fructose-streptozotocin-induced diabetic cardiac dysfunction in rats

- Endocrine Research
- The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes
- Cheol Ryong Ku, Yoon Hee Cho, Zhen-Yu Hong, Ha Lee, Sue Ji Lee, Seung-soo Hong, Eun Jig Lee
- Endocrinol Metab. 2016;31(2):328-335. Published online April 8, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.328
- 4,132 View
- 50 Download
- 25 Web of Science
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Resveratrol (RSV) is a polyphenolic phytoalexin that has many effects on metabolic diseases such as diabetes and obesity. Given the importance of brown adipose tissue (BAT) for energy expenditure, we investigated the effects of RSV on brown adipocytes.
Methods For the
in vitro study, interscapular BAT was isolated from 7-week-old male Sprague Dawley rats. For thein vivo study, 7-week-old male Otsuka Long Evans Tokushima Fatty (OLETF) rats were divided into four groups and treated for 27 weeks with: standard diet (SD); SD+RSV (10 mg/kg body weight, daily); high fat diet (HFD); HFD+RSV. RSV was provided via oral gavage once daily during thein vivo experiments.Results RSV treatment of primary cultured brown preadipocytes promoted mitochondrial activity, along with over-expression of estrogen receptor α (ER-α). In OLETF rats, both HFD and RSV treatment increased the weight of BAT and the differentiation of BAT. However, only RSV increased the mitochondrial activity and ER-α expression of BAT in the HFD-fed group. Finally, RSV improved the insulin sensitivity of OLETF rats by increasing the mitochondrial activity of BAT, despite having no effects on white adipocytes and muscles in either diet group.
Conclusion RSV could improve insulin resistance, which might be associated with mitochondrial activity of brown adipocyte. Further studies evaluating the activity of RSV for both the differentiation and mitochondrial activity of BAT could be helpful in investigating the effects of RSV on metabolic parameters.
-
Citations
Citations to this article as recorded by- Natural Bioactive Compounds from Foods Inhibited Pigmentation Especially Potential Application of Fucoxanthin to Chloasma: a Mini-Review
Yida Wang, Hang Qi
Food Reviews International.2024; 40(1): 20. CrossRef - Resveratrol combats chronic diseases through enhancing mitochondrial quality
Weichu Tao, Hu Zhang, Xia Jiang, Ning Chen
Food Science and Human Wellness.2024; 13(2): 597. CrossRef - The Potential to Fight Obesity with Adipogenesis Modulating Compounds
Jiaqi Zhao, Ailin Zhou, Wei Qi
International Journal of Molecular Sciences.2022; 23(4): 2299. CrossRef - Macrophage and Adipocyte Mitochondrial Dysfunction in Obesity-Induced Metabolic Diseases
Liwen Wang, Jie Hu, Haiyan Zhou
The World Journal of Men's Health.2021; 39(4): 606. CrossRef - Precision Nutrition to Activate Thermogenesis as a Complementary Approach to Target Obesity and Associated-Metabolic-Disorders
Marina Reguero, Marta Gómez de Cedrón, Sonia Wagner, Guillermo Reglero, José Carlos Quintela, Ana Ramírez de Molina
Cancers.2021; 13(4): 866. CrossRef - Natural Antioxidant Application on Fat Accumulation: Preclinical Evidence
Proshanta Roy, Daniele Tomassoni, Enea Traini, Ilenia Martinelli, Maria Vittoria Micioni Di Bonaventura, Carlo Cifani, Francesco Amenta, Seyed Khosrow Tayebati
Antioxidants.2021; 10(6): 858. CrossRef - Activation of Brown Adipose Tissue and Promotion of White Adipose Tissue Browning by Plant-based Dietary Components in Rodents: A Systematic Review
Francisco J Osuna-Prieto, Borja Martinez-Tellez, Antonio Segura-Carretero, Jonatan R Ruiz
Advances in Nutrition.2021; 12(6): 2147. CrossRef - Role of Dietary Polyphenols in Adipose Tissue Browning: A Narrative Review
Juan Salazar, Clímaco Cano, José L. Pérez, Ana Castro, María P. Díaz, Bermary Garrido, Rubén Carrasquero, Maricarmen Chacín, Manuel Velasco, Luis D´Marco, Joselyn Rojas-Quintero, Valmore Bermúdez
Current Pharmaceutical Design.2020; 26(35): 4444. CrossRef - Brown and Brite: The Fat Soldiers in the Anti-obesity Fight
Shireesh Srivastava, Richard L. Veech
Frontiers in Physiology.2019;[Epub] CrossRef - Effect of resveratrol on adipokines and myokines involved in fat browning: Perspectives in healthy weight against obesity
Oh Yoen Kim, Ji Yeon Chung, Juhyun Song
Pharmacological Research.2019; 148: 104411. CrossRef - Ginsenoside Rb2 Alleviates Obesity by Activation of Brown Fat and Induction of Browning of White Fat
Yilian Hong, Yi Lin, Qiya Si, Lijuan Yang, Weisong Dong, Xuejiang Gu
Frontiers in Endocrinology.2019;[Epub] CrossRef - Programming of the Beige Phenotype in White Adipose Tissue of Adult Mice by Mild Resveratrol and Nicotinamide Riboside Supplementations in Early Postnatal Life
Alba Serrano, Madhu Asnani-Kishnani, Ana María Rodríguez, Andreu Palou, Joan Ribot, María Luisa Bonet
Molecular Nutrition & Food Research.2018;[Epub] CrossRef - Effects of Polyphenols on Thermogenesis and Mitochondrial Biogenesis
Tanila Wood dos Santos, Quélita Cristina Pereira, Lucimara Teixeira, Alessandra Gambero, Josep A. Villena, Marcelo Lima Ribeiro
International Journal of Molecular Sciences.2018; 19(9): 2757. CrossRef - Programming mediated by fatty acids affects uncoupling protein 1 (UCP-1) in brown adipose tissue
Perla P. Argentato, Helena de Cássia César, Débora Estadella, Luciana P. Pisani
British Journal of Nutrition.2018; 120(6): 619. CrossRef - Effects of Genistein on Differentiation and Viability of Human Visceral Adipocytes
Elena Grossini, Serena Farruggio, Giulia Raina, David Mary, Giacomo Deiro, Sergio Gentilli
Nutrients.2018; 10(8): 978. CrossRef - A comprehensive review of the health perspectives of resveratrol
Abdur Rauf, Muhammad Imran, Hafiz Ansar Rasul Suleria, Bashir Ahmad, Dennis G. Peters, Mohammad S. Mubarak
Food & Function.2017; 8(12): 4284. CrossRef - The Role of Circulating Slit2, the One of the Newly Batokines, in Human Diabetes Mellitus
Yea Eun Kang, Sorim Choung, Ju Hee Lee, Hyun Jin Kim, Bon Jeong Ku
Endocrinology and Metabolism.2017; 32(3): 383. CrossRef - A nutritional perspective on UCP1-dependent thermogenesis
M. Luisa Bonet, Josep Mercader, Andreu Palou
Biochimie.2017; 134: 99. CrossRef - The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity
Yueshui Zhao, Bo Chen, Jing Shen, Lin Wan, Yinxin Zhu, Tao Yi, Zhangang Xiao
Oxidative Medicine and Cellular Longevity.2017; 2017: 1. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - Antiobesity effects of resveratrol: which tissues are involved?
Alfredo Fernández‐Quintela, Iñaki Milton‐Laskibar, Marcela González, Maria P. Portillo
Annals of the New York Academy of Sciences.2017; 1403(1): 118. CrossRef - Resveratrol attenuates triglyceride accumulation associated with upregulation of Sirt1 and lipoprotein lipase in 3T3-L1 adipocytes
Haruki Imamura, Daiji Nagayama, Noriko Ishihara, Syo Tanaka, Rena Watanabe, Yasuhiro Watanabe, Yuta Sato, Takashi Yamaguchi, Noriko Ban, Hidetoshi Kawana, Masahiro Ohira, Kei Endo, Atsuhito Saiki, Kohji Shirai, Ichiro Tatsuno
Molecular Genetics and Metabolism Reports.2017; 12: 44. CrossRef - Resveratrol has dose-dependent effects on DNA fragmentation and mitochondrial activity of ovine secondary follicles cultured in vitro
T.J.S. Macedo, V.R.P. Barros, A.P.O. Monte, B.B. Gouveia, M.É.S. Bezerra, A.Y.P. Cavalcante, R.S. Barberino, V.G. Menezes, M.H.T. Matos
Zygote.2017; 25(4): 434. CrossRef - Response: The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes (Endocrinol Metab2016;31:328-35, Cheol Ryong Ku et al.)
Cheol Ryong Ku, Eun Jig Lee
Endocrinology and Metabolism.2016; 31(3): 482. CrossRef - Letter: The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes (Endocrinol Metab2016;31:328-35, Cheol Ryong Ku et al.)
Ji-Young Cha
Endocrinology and Metabolism.2016; 31(3): 480. CrossRef
- Natural Bioactive Compounds from Foods Inhibited Pigmentation Especially Potential Application of Fucoxanthin to Chloasma: a Mini-Review

- Mechanisms of Vascular Calcification: The Pivotal Role of Pyruvate Dehydrogenase Kinase 4
- Jaechan Leem, In-Kyu Lee
- Endocrinol Metab. 2016;31(1):52-61. Published online March 16, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.1.52
- 4,433 View
- 69 Download
- 30 Web of Science
- 28 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Vascular calcification, abnormal mineralization of the vessel wall, is frequently associated with aging, atherosclerosis, diabetes mellitus, and chronic kidney disease. Vascular calcification is a key risk factor for many adverse clinical outcomes, including ischemic cardiac events and subsequent cardiovascular mortality. Vascular calcification was long considered to be a passive degenerative process, but it is now recognized as an active and highly regulated process similar to bone formation. However, despite numerous studies on the pathogenesis of vascular calcification, the mechanisms driving this process remain poorly understood. Pyruvate dehydrogenase kinases (PDKs) play an important role in the regulation of cellular metabolism and mitochondrial function. Recent studies show that PDK4 is an attractive therapeutic target for the treatment of various metabolic diseases. In this review, we summarize our current knowledge regarding the mechanisms of vascular calcification and describe the role of PDK4 in the osteogenic differentiation of vascular smooth muscle cells and development of vascular calcification. Further studies aimed at understanding the molecular mechanisms of vascular calcification will be critical for the development of novel therapeutic strategies.
-
Citations
Citations to this article as recorded by- Gamut of glycolytic enzymes in vascular smooth muscle cell proliferation: Implications for vascular proliferative diseases
Ankan Sarkar, Sandip V. Pawar, Kanwaljit Chopra, Manish Jain
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2024; 1870(3): 167021. CrossRef - MAPK14 as a key gene for regulating inflammatory response and macrophage M1 polarization induced by ferroptotic keratinocyte in psoriasis
Lin Zhou, Yingdong Zhong, Chaowei Li, Yu Zhou, Xi Liu, Lincai Li, Zhengwei Zou, Zhihui Zhong, Junsong Ye
Inflammation.2024;[Epub] CrossRef - Pyruvate dehydrogenase kinase 4 promotes osteoblastic potential of BMP9 by boosting Wnt/β-catenin signaling in mesenchymal stem cells
Yuan-Yuan Yang, Hong-Hong Luo, Yi-Xuan Deng, Xin-Tong Yao, Jie Zhang, Yu-Xi Su, Bai-Cheng He
The International Journal of Biochemistry & Cell Biology.2023; 154: 106341. CrossRef - lncRNA MEG3 Promotes PDK4/GSK-3β/β-Catenin Axis in MEFs by Targeting miR-532-5p
Yuan-Yuan Yang, Yi-Xuan Deng, Xin-Tong Yao, Hong-Hong Luo, Wen-Ge He, Xuan-Ling Cao, Rong-Chun Chen, Bai-Cheng He, Hai-Tao Jiang, Jing Wang, Sedat Kacar
Oxidative Medicine and Cellular Longevity.2023; 2023: 1. CrossRef - Mitochondrial dynamics in vascular remodeling and target-organ damage
Tong Zhu, Qingxun Hu, Yanggang Yuan, Huijuan Yao, Jian Zhang, Jia Qi
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - PDK4-dependent hypercatabolism and lactate production of senescent cells promotes cancer malignancy
Xuefeng Dou, Qiang Fu, Qilai Long, Shuning Liu, Yejun Zou, Da Fu, Qixia Xu, Zhirui Jiang, Xiaohui Ren, Guilong Zhang, Xiaoling Wei, Qingfeng Li, Judith Campisi, Yuzheng Zhao, Yu Sun
Nature Metabolism.2023; 5(11): 1887. CrossRef - Identification of PDK4 as Hub Gene for Diabetic Nephropathy Using Co-Expression Network Analysis
Yuanyuan Han, Liangzi Jin, Liangzhi Wang, Lan Wei, Chao Tu
Kidney and Blood Pressure Research.2023; 48(1): 522. CrossRef - Flavocoxid Ameliorates Aortic Calcification Induced by Hypervitaminosis D3 and Nicotine in Rats Via Targeting TNF-α, IL-1β, iNOS, and Osteogenic Runx2
Ahmed E. Amer, George S. G. Shehatou, Hassan A. El-Kashef, Manar A. Nader, Ahmed R. El-Sheakh
Cardiovascular Drugs and Therapy.2022; 36(6): 1047. CrossRef - Diabetic mellitus, vascular calcification and hypoxia: A complex and neglected tripartite relationship
Xue-Jiao Sun, Nai-Feng Liu
Cellular Signalling.2022; 91: 110219. CrossRef - Insights Into the Role of Mitochondria in Vascular Calcification
ZL Zeng, Qing Yuan, Xuyu Zu, Jianghua Liu
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Induced pluripotent stem cell-derived smooth muscle cells to study cardiovascular calcification
Samantha K. Atkins, Abhijeet R. Sonawane, Romi Brouwhuis, Johana Barrientos, Anna Ha, Maximillian Rogers, Takeshi Tanaka, Takehito Okui, Shiori Kuraoka, Sasha A. Singh, Masanori Aikawa, Elena Aikawa
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Phenotypic plasticity of vascular smooth muscle cells in vascular calcification: Role of mitochondria
Yan Zhong Liu, Zong Xiang Li, Lin Lin Zhang, Dan Wang, Yi Ping Liu
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Febuxostat attenuates vascular calcification induced by vitamin D3 plus nicotine in rats
Ahmed E. Amer, Ahmed R. El-Sheakh, Mohamed F. Hamed, Hassan A. El-Kashef, Manar A. Nader, George S.G. Shehatou
European Journal of Pharmaceutical Sciences.2021; 156: 105580. CrossRef - Mitochondria and traffic-related air pollution linked coronary artery calcification: exploring the missing link
Bhavana Sivakumar, Gino A. Kurian
Reviews on Environmental Health.2021; 36(4): 545. CrossRef - Mitochondria Homeostasis and Vascular Medial Calcification
Min li, Yi Zhu, Sandip Kumar Jaiswal, Nai-Feng Liu
Calcified Tissue International.2021; 109(2): 113. CrossRef - POSTN promotes diabetic vascular calcification by interfering with autophagic flux
Xue-Jiao Sun, Wen-Qi Ma, Yi Zhu, Nai-Feng Liu
Cellular Signalling.2021; 83: 109983. CrossRef - The MAMs Structure and Its Role in Cell Death
Nan Wang, Chong Wang, Hongyang Zhao, Yichun He, Beiwu Lan, Liankun Sun, Yufei Gao
Cells.2021; 10(3): 657. CrossRef - Pyruvate dehydrogenase kinases (PDKs): an overview toward clinical applications
Xiuxiu Wang, Xiaoyue Shen, Yuting Yan, Hongmin Li
Bioscience Reports.2021;[Epub] CrossRef - Label-Free Multiphoton Microscopy for the Detection and Monitoring of Calcific Aortic Valve Disease
Ishita Tandon, Kyle P. Quinn, Kartik Balachandran
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - Vascular Calcification—New Insights into Its Mechanism
Sun Joo Lee, In-Kyu Lee, Jae-Han Jeon
International Journal of Molecular Sciences.2020; 21(8): 2685. CrossRef - Osteocalcin Regulates Arterial Calcification Via Altered Wnt Signaling and Glucose Metabolism
Nabil A Rashdan, Alisia M Sim, Lin Cui, Kanchan Phadwal, Fiona L Roberts, Roderick Carter, Derya D Ozdemir, Peter Hohenstein, John Hung, Jakub Kaczynski, David E Newby, Andrew H Baker, Gerard Karsenty, Nicholas M Morton, Vicky E MacRae
Journal of Bone and Mineral Research.2020; 35(2): 357. CrossRef - The role of mitochondria in vascular calcification
Pengbo Wang, Naijin Zhang, Boquan Wu, Shaojun Wu, Ying Zhang, Yingxian Sun
Journal of Translational Internal Medicine.2020; 8(2): 80. CrossRef - Cerebral blood flow in dystonia due to pantothenate kinase-associated neurodegeneration
Peter Stoeter, Pedro Roa-Sanchez, Cesar F Gonzalez, Herwin Speckter, Jairo Oviedo, Pamela Bido
The Neuroradiology Journal.2020; 33(6): 479. CrossRef - PDK4 promotes vascular calcification by interfering with autophagic activity and metabolic reprogramming
Wen-Qi Ma, Xue-Jiao Sun, Yi Zhu, Nai-Feng Liu
Cell Death & Disease.2020;[Epub] CrossRef - Restoring mitochondrial biogenesis with metformin attenuates β-GP-induced phenotypic transformation of VSMCs into an osteogenic phenotype via inhibition of PDK4/oxidative stress-mediated apoptosis
Wen-Qi Ma, Xue-Jiao Sun, Ying Wang, Yi Zhu, Xi-Qiong Han, Nai-Feng Liu
Molecular and Cellular Endocrinology.2019; 479: 39. CrossRef - Salusin-β Promotes Vascular Calcification via Nicotinamide Adenine Dinucleotide Phosphate/Reactive Oxygen Species-Mediated Klotho Downregulation
Haijian Sun, Feng Zhang, Yu Xu, Shuo Sun, Huiping Wang, Qiong Du, Chenxin Gu, Stephen M. Black, Ying Han, Haiyang Tang
Antioxidants & Redox Signaling.2019; 31(18): 1352. CrossRef - Fibroblast Growth Factor 21 (FGF21) Promotes Formation of Aerobic Myofibers via the FGF21‐SIRT1‐AMPK‐PGC1α Pathway
Xinyi Liu, Yongliang Wang, Liming Hou, Yuanzhu Xiong, Shuhong Zhao
Journal of Cellular Physiology.2017; 232(7): 1893. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- Gamut of glycolytic enzymes in vascular smooth muscle cell proliferation: Implications for vascular proliferative diseases

- Endocrine Research
- Selective Mitochondrial Uptake of MKT-077 Can Suppress Medullary Thyroid Carcinoma Cell Survival In Vitro and In Vivo
- Dmytro Starenki, Jong-In Park
- Endocrinol Metab. 2015;30(4):593-603. Published online December 31, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.4.593
- 3,894 View
- 39 Download
- 16 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor mainly caused by mutations in the rearranged during transfection (
RET ) proto-oncogene. Not all patients with progressive MTC respond to current therapy inhibiting RET, demanding additional therapeutic strategies. We recently demonstrated that disrupting mitochondrial metabolism using a mitochondria-targeted agent or by depleting a mitochondrial chaperone effectively suppressed human MTC cells in culture and in mouse xenografts by inducing apoptosis and RET downregulation. These observations led us to hypothesize that mitochondria are potential therapeutic targets for MTC. This study further tests this hypothesis using1-ethyl-2-[[3-ethyl-5-(3-methylbenzothiazolin-2-yliden)]-4-oxothiazolidin-2-ylidenemethyl] pyridinium chloride (MKT-077), a water-soluble rhodocyanine dye analogue, which can selectively accumulate in mitochondria.Methods The effects of MKT-077 on cell proliferation, survival, expression of RET and tumor protein 53 (TP53), and mitochondrial activity were determined in the human MTC lines in culture and in mouse xenografts.
Results MKT-077 induced cell cycle arrest in TT and MZ-CRC-1. Intriguingly, MKT-077 also induced RET downregulation and strong cell death responses in TT cells, but not in MZ-CRC-1 cells. This discrepancy was mainly due to the difference between the capacities of these cell lines to retain MKT-077 in mitochondria. The cytotoxicity of MKT-077 in TT cells was mainly attributed to oxidative stress while being independent of TP53. MKT-077 also effectively suppressed tumor growth of TT xenografts.
Conclusion MKT-077 can suppress cell survival of certain MTC subtypes by accumulating in mitochondria and interfering with mitochondrial activity although it can also suppress cell proliferation via other mechanisms. These results consistently support the hypothesis that mitochondrial targeting has therapeutic potential for MTC.
-
Citations
Citations to this article as recorded by- Immunoexpression of HSPA9 and CUL2 in prostatic tissue and adenocarcinoma
Carlos Gustavo Hirth, Gislane Rocha Vasconcelos, Maria do Perpétuo Socorro Saldanha da Cunha, Carlos Heli Bezerra Leite, Conceição Aparecida Dornelas
Annals of Diagnostic Pathology.2022; 56: 151843. CrossRef - Analogs of the Heat Shock Protein 70 Inhibitor MKT-077 Suppress Medullary Thyroid Carcinoma Cells
Seung-Keun Hong, Dmytro Starenki, Oleta T. Johnson, Jason E. Gestwicki, Jong-In Park
International Journal of Molecular Sciences.2022; 23(3): 1063. CrossRef - Effect of F16-Betulin Conjugate on Mitochondrial Membranes and Its Role in Cell Death Initiation
Mikhail V. Dubinin, Alena A. Semenova, Darya A. Nedopekina, Eldar V. Davletshin, Anna Yu. Spivak, Konstantin N. Belosludtsev
Membranes.2021; 11(5): 352. CrossRef - A BAG's life: Every connection matters in cancer
Elena Mariotto, Giampietro Viola, Carlo Zanon, Sanja Aveic
Pharmacology & Therapeutics.2020; 209: 107498. CrossRef - Emerging insights into mitochondria-specific targeting and drug delivering strategies: Recent milestones and therapeutic implications
Sugapriya Dhanasekaran, Divya Venugopal, Noura Al-Dayan, Vijaya Ravinayagam, Arif Ahmed Mohammed
Saudi Journal of Biological Sciences.2020; 27(12): 3581. CrossRef - Growth Inhibitory Signaling of the Raf/MEK/ERK Pathway
Pui-Kei Wu, Andrew Becker, Jong-In Park
International Journal of Molecular Sciences.2020; 21(15): 5436. CrossRef - Heat Shock Proteins (HSPs): A Novel Target for Cancer Metastasis Prevention
Vinayak Narayanankutty , Arunaksharan Narayanankutty, Anusree Nair
Current Drug Targets.2019; 20(7): 727. CrossRef - Токсичність МАРК у карциномах щитоподібної залози. Механізми пригнічення сигнального каскаду (огляд літератури та власних даних)
B. B. Guda, V. V. Pushkarev, O. I. Kovzun, V. P. Pushkarev, M. D. Tronko
Шпитальна хірургія. Журнал імені Л. Я. Ковальчука.2019; (3): 84. CrossRef - Mortalin (GRP75/HSPA9) Promotes Survival and Proliferation of Thyroid Carcinoma Cells
Dmytro Starenki, Nadiya Sosonkina, Seung-Keun Hong, Ricardo V. Lloyd, Jong-In Park
International Journal of Molecular Sciences.2019; 20(9): 2069. CrossRef - Mitochondrial autophagosomes as a mechanism of drug resistance in breast carcinoma
Ayman N. Abunimer, Heba Mohammed, Katherine L. Cook, David R. Soto-Pantoja, Maria Mercedes Campos, Mones S. Abu-Asab
Ultrastructural Pathology.2018; 42(2): 170. CrossRef - Genotoxic Responses of Mitochondrial Oxygen Consumption Rate and Mitochondrial Semiquinone Radicals in Tumor Cells
Kumiko Yamamoto, Hironobu Yasui, Tomoki Bo, Tohru Yamamori, Wakako Hiraoka, Toshihide Yamasaki, Ken-ichi Yamada, Osamu Inanami
Applied Magnetic Resonance.2018; 49(8): 837. CrossRef - Suppression of B-RafV600E melanoma cell survival by targeting mitochondria using triphenyl-phosphonium-conjugated nitroxide or ubiquinone
Seung-Keun Hong, Dmytro Starenki, Pui-Kei Wu, Jong-In Park
Cancer Biology & Therapy.2017; 18(2): 106. CrossRef - Mitochondria chaperone GRP75 moonlighting as a cell cycle controller to derail endocytosis provides an opportunity for nanomicrosphere intracellular delivery
Zhihui Gao, Xiuran Niu, Qing Zhang, Hang Chen, Aiai Gao, Shanshan Qi, Rong Xiang, Mattias Belting, Sihe Zhang
Oncotarget.2017; 8(35): 58536. CrossRef - Targeted Therapy for Medullary Thyroid Cancer: A Review
S. R. Priya, Chandra Shekhar Dravid, Raghunadharao Digumarti, Mitali Dandekar
Frontiers in Oncology.2017;[Epub] CrossRef - Vandetanib and cabozantinib potentiate mitochondria-targeted agents to suppress medullary thyroid carcinoma cells
Dmytro Starenki, Seung-Keun Hong, Pui-Kei Wu, Jong-In Park
Cancer Biology & Therapy.2017; 18(7): 473. CrossRef
- Immunoexpression of HSPA9 and CUL2 in prostatic tissue and adenocarcinoma

- Endocrine Research
- Diastolic Dysfunction Induced by a High-Fat Diet Is Associated with Mitochondrial Abnormality and Adenosine Triphosphate Levels in Rats
- Ki-Woon Kang, Ok-Soon Kim, Jung Yeon Chin, Won Ho Kim, Sang Hyun Park, Yu Jeong Choi, Jong Ho Shin, Kyung Tae Jung, Do-Seon Lim, Seong-Kyu Lee
- Endocrinol Metab. 2015;30(4):557-568. Published online December 31, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.4.557
- 4,879 View
- 46 Download
- 13 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Obesity is well-known as a risk factor for heart failure, including diastolic dysfunction. However, this mechanism in high-fat diet (HFD)-induced obese rats remain controversial. The purpose of this study was to investigate whether cardiac dysfunction develops when rats are fed with a HFD for 10 weeks; additionally, we sought to investigate the association between mitochondrial abnormalities, adenosine triphosphate (ATP) levels and cardiac dysfunction.
Methods We examined myocardia in Wistar rats after 10 weeks of HFD (45 kcal% fat,
n =6) or standard diet (SD, n=6). Echocardiography, histomorphologic analysis, and electron microscopy were performed. The expression levels of mitochondrial oxidative phosphorylation (OXPHOS) subunit genes, peroxisome-proliferator-activated receptor γ co-activator-1α (PGC1α) and anti-oxidant enzymes were assessed. Markers of oxidative stress damage, mitochondrial DNA copy number and myocardial ATP level were also examined.Results After 10 weeks, the body weight of the HFD group (349.6±22.7 g) was significantly higher than that of the SD group (286.8±14.9 g), and the perigonadal and epicardial fat weights of the HFD group were significantly higher than that of the SD group. Histomorphologic and electron microscopic images were similar between the two groups. However, in the myocardium of the HFD group, the expression levels of OXPHOS subunit NDUFB5 in complex I and PGC1α, and the mitochondrial DNA copy number were decreased and the oxidative stress damage marker 8-hydroxydeoxyguanosine was increased, accompanied by reduced ATP levels.
Conclusion Diastolic dysfunction was accompanied by the mitochondrial abnormality and reduced ATP levels in the myocardium of 10 weeks-HFD-induced rats.
-
Citations
Citations to this article as recorded by- Epicardial Adipose Tissue in Myocardial Disease: From Physiology to Heart Failure Phenotypes
Alexios S. Antonopoulos, Charalampos Papastamos, Dennis V. Cokkinos, Konstantinos Tsioufis, Dimitris Tousoulis
Current Problems in Cardiology.2023; 48(10): 101841. CrossRef - Metabolic mitochondrial alterations prevail in the female rat heart 8 weeks after exercise cessation
Carolina Tocantins, João D. Martins, Óscar M. Rodrigues, Luís F. Grilo, Mariana S. Diniz, Jelena Stevanovic‐Silva, Jorge Beleza, Pedro Coxito, David Rizo‐Roca, Estela Santos‐Alves, Manoel Rios, Lina Carvalho, António J. Moreno, António Ascensão, José Maga
European Journal of Clinical Investigation.2023;[Epub] CrossRef - The link between obesity and aging - insights into cardiac energy metabolism
Patricia Owesny, Tilman Grune
Mechanisms of Ageing and Development.2023; 216: 111870. CrossRef - Surgically Metabolic Resection of Pericardial Fat to Ameliorate Myocardial Mitochondrial Dysfunction in Acute Myocardial Infarction Obese Rats
Ki-Woon Kang, Ju-Young Ko, Hyunghee Lee, Seung Yong Shin, Wang Soo Lee, Joonhwa Hong, Sang-Wook Kim, Seong-Kyu Lee, Min-Ho Oak
Journal of Korean Medical Science.2022;[Epub] CrossRef - Cilostazol attenuates cardiac oxidative stress and inflammation in hypercholesterolemic rats
Rosane de Oliveira Lopes, Gabriel Ferreira Lima, Ana Beatriz Araújo Mendes, Lis Jappour Autran, Nikolas Cunha de Assis Pereira, Stephani Correia Brazão, Beatriz Alexandre-Santos, Eliete Dalla Corte Frantz, Christianne Brêtas Vieira Scaramello, Fernanda Ca
Naunyn-Schmiedeberg's Archives of Pharmacology.2022; 395(7): 789. CrossRef - The impact of diet upon mitochondrial physiology (Review)
Ioannis Kyriazis, Eleni Vassi, Maria Alvanou, Christos Angelakis, Zoi Skaperda, Fotios Tekos, Venkata Garikipati, Demetrios Spandidos, Demetrios Kouretas
International Journal of Molecular Medicine.2022;[Epub] CrossRef - SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways
Yasser Majeed, Najeeb Halabi, Aisha Y. Madani, Rudolf Engelke, Aditya M. Bhagwat, Houari Abdesselem, Maha V. Agha, Muneera Vakayil, Raphael Courjaret, Neha Goswami, Hisham Ben Hamidane, Mohamed A. Elrayess, Arash Rafii, Johannes Graumann, Frank Schmidt, N
Scientific Reports.2021;[Epub] CrossRef - Physical Exercise Potentially Targets Epicardial Adipose Tissue to Reduce Cardiovascular Disease Risk in Patients with Metabolic Diseases: Oxidative Stress and Inflammation Emerge as Major Therapeutic Targets
Thembeka A. Nyawo, Carmen Pheiffer, Sithandiwe E. Mazibuko-Mbeje, Sinenhlanhla X. H. Mthembu, Tawanda M. Nyambuya, Bongani B. Nkambule, Hanél Sadie-Van Gijsen, Hans Strijdom, Luca Tiano, Phiwayinkosi V. Dludla
Antioxidants.2021; 10(11): 1758. CrossRef - Potentially Critical Roles ofNDUFB5,TIMMDC1,and VDAC3in the Progression of Septic Cardiomyopathy Through Integrated Bioinformatics Analysis
Kai Kang, Jingtian Li, Ruidong Li, Xiufeng Xu, Jianli Liu, Limin Qin, Tao Huang, Jinhua Wu, Min Jiao, Miaomiao Wei, Hongjie Wang, Tao Wang, Quan Zhang
DNA and Cell Biology.2020; 39(1): 105. CrossRef - Liraglutide shows superior cardiometabolic benefits than lorcaserin in a novel free choice diet-induced obese rat model
François Briand, Emmanuel Brousseau, Julie Maupoint, Caroline Dubroca, Clément Costard, Natalia Breyner, Rémy Burcelin, Thierry Sulpice
European Journal of Pharmacology.2020; 882: 173316. CrossRef - Mitochondrial impairment following neonatal overfeeding: A comparison between normal and ischemic‐reperfused hearts
Cristiane de Moura Freitas, Luciana Caroline Paulino do Nascimento, Glauber Rudá Feitoza Braz, Severina Cassia Andrade‐Silva, Nelson C. Lima‐Junior, Tercya de Araujo Silva, Mariana Pinheiro Fernandes, Diorginis José Soares Ferreira, Claudia Jacques Lagran
Journal of Cellular Biochemistry.2019; 120(5): 7341. CrossRef - Exercise training reverses age‐induced diastolic dysfunction and restores coronary microvascular function
Kazuki Hotta, Bei Chen, Bradley J. Behnke, Payal Ghosh, John N. Stabley, Jeremy A. Bramy, Jaime L. Sepulveda, Michael D. Delp, Judy M. Muller‐Delp
The Journal of Physiology.2017; 595(12): 3703. CrossRef - Qiliqiangxin Enhances Cardiac Glucose Metabolism and Improves Diastolic Function in Spontaneously Hypertensive Rats
Jingfeng Wang, Zhiming Li, Yanyan Wang, Jingjing Zhang, Weipeng Zhao, Mingqiang Fu, Xueting Han, Jingmin Zhou, Junbo Ge
Evidence-Based Complementary and Alternative Medicine.2017; 2017: 1. CrossRef
- Epicardial Adipose Tissue in Myocardial Disease: From Physiology to Heart Failure Phenotypes

- Obesity and Metabolism
- Glucagon-Like Peptide-1 Increases Mitochondrial Biogenesis and Function in INS-1 Rat Insulinoma Cells
- Mi Yeon Kang, Tae Jung Oh, Young Min Cho
- Endocrinol Metab. 2015;30(2):216-220. Published online June 30, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.2.216
- 4,003 View
- 44 Download
- 41 Web of Science
- 40 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Glucagon-like peptide-1 (GLP-1) is a gut-derived incretin hormone that increases glucose-stimulated insulin secretion in pancreatic β-cells. Since mitochondrial function is crucial to insulin secretion, we hypothesized that GLP-1 may increase mitochondrial biogenesis in pancreatic β-cells. We treated INS-1 rat insulinoma cells with GLP-1 or exendin-4 for 48 hours and measured mitochondrial mass and function. Both GLP-1 and exendin-4 increased mitochondrial mass by approximately 20%. The mitochondria/cytosol ratio was increased from 7.60±3.12% to 10.53±2.70% by exendin-4. In addition, GLP-1 increased the mitochondrial membrane potential and oxygen consumption. Proliferator-activated receptor-gamma coactivator 1α expression was increased approximately 2-fold by GLP-1 treatment. In conclusion, the present study presents evidence for a new mechanism of action by which GLP-1 improves pancreatic β-cell function via enhanced mitochondrial mass and performance.
-
Citations
Citations to this article as recorded by- New fraternine analogues: Evaluation of the antiparkinsonian effect in the model of Parkinson's disease
Andréia Biolchi Mayer, Henrique de Oliveira Amaral, Danilo Gustavo R. de Oliveira, Gabriel Avohay Alves Campos, Priscilla Galante Ribeiro, Solange Cristina Rego Fernandes, Adolfo Carlos Barros de Souza, Raffael Júnio Araújo de Castro, Anamélia Lorenzetti
Neuropeptides.2024; 103: 102390. CrossRef - Prospects of antidiabetic drugs in the treatment of neurodegenerative disease
Lidan Hu, Wenmin Wang, Xiangjun Chen, Guannan Bai, Liangjian Ma, Xin Yang, Qiang Shu, Xuekun Li
Brain‐X.2024;[Epub] CrossRef - Attenuating mitochondrial dysfunction and morphological disruption with PT320 delays dopamine degeneration in MitoPark mice
Vicki Wang, Kuan-Yin Tseng, Tung-Tai Kuo, Eagle Yi-Kung Huang, Kuo-Lun Lan, Zi-Rong Chen, Kuo-Hsing Ma, Nigel H. Greig, Jin Jung, Ho-II Choi, Lars Olson, Barry J. Hoffer, Yuan-Hao Chen
Journal of Biomedical Science.2024;[Epub] CrossRef - Liraglutide demonstrates a therapeutic effect on mitochondrial dysfunction in human SGBS adipocytes in vitro
Maija Vaittinen, Mariana Ilha, Elena Herbers, Anita Wagner, Kirsi A. Virtanen, Kirsi H. Pietiläinen, Eija Pirinen, Jussi Pihlajamäki
Diabetes Research and Clinical Practice.2023; 199: 110635. CrossRef - The cardioprotective effect of human glucagon‐like peptide‐1 receptor agonist (semaglutide) on cisplatin‐induced cardiotoxicity in rats: Targeting mitochondrial functions, dynamics, biogenesis, and redox status pathways
Marwa Mohamed Atef, Yasser Mostafa Hafez, Omnia Safwat El‐Deeb, Eman H. Basha, Radwa Ismail, Hanan Alshenawy, Rasha Osama El‐Esawy, Amira Kamel Eltokhy
Cell Biochemistry and Function.2023; 41(4): 450. CrossRef - Targeting mitochondrial quality control for diabetic cardiomyopathy: Therapeutic potential of hypoglycemic drugs
Yutong Zhou, Wendong Suo, Xinai Zhang, Jiaojiao Liang, Weizhe Zhao, Yue Wang, Hong Li, Qing Ni
Biomedicine & Pharmacotherapy.2023; 168: 115669. CrossRef - Soft X-ray tomography to map and quantify organelle interactions at the mesoscale
Valentina Loconte, Jitin Singla, Angdi Li, Jian-Hua Chen, Axel Ekman, Gerry McDermott, Andrej Sali, Mark Le Gros, Kate L. White, Carolyn A. Larabell
Structure.2022; 30(4): 510. CrossRef - Comparisons of pleiotropic effects of SGLT2 inhibition and GLP-1 agonism on cardiac glucose intolerance in heart dysfunction
Belma Turan, Aysegul Durak, Yusuf Olgar, Erkan Tuncay
Molecular and Cellular Biochemistry.2022; 477(11): 2609. CrossRef - Modulation of Reactive Oxygen Species Homeostasis as a Pleiotropic Effect of Commonly Used Drugs
Carolin Thomas, Lia Wurzer, Ernst Malle, Michael Ristow, Corina T. Madreiter-Sokolowski
Frontiers in Aging.2022;[Epub] CrossRef - Anti-cancer effects of sitagliptin, vildagliptin, and exendin-4 on triple-negative breast cancer cells via mitochondrial modulation
POOJA JAISWAL, VERSHA TRIPATHI, ANSHUL ASSAIYA, DHARMENDRA KASHYAP, RAHUL DUBEY, ANAMIKA SINGH, JANESH KUMAR, HEM CHANDRA JHA, RAJESH SHARMA, AMIT KUMAR DIXIT, HAMENDRA SINGH PARMAR
BIOCELL.2022; 46(12): 2645. CrossRef - Effect of liraglutide on neural and peripheral markers of metabolic function during antipsychotic treatment in rats
Ilijana Babic, Dominic Sellers, Paul L Else, Jessica Nealon, Ashleigh L Osborne, Nagesh Pai, Katrina Weston-Green
Journal of Psychopharmacology.2021; 35(3): 284. CrossRef - Sustained Release GLP-1 Agonist PT320 Delays Disease Progression in a Mouse Model of Parkinson’s Disease
Vicki Wang, Tung-Tai Kuo, Eagle Yi-Kung Huang, Kuo-Hsing Ma, Yu-Ching Chou, Zhao-Yang Fu, Li-Wen Lai, Jin Jung, Hoi-II Choi, Doo-Sup Choi, Yazhou Li, Lars Olson, Nigel H. Greig, Barry J. Hoffer, Yuan-Hao Chen
ACS Pharmacology & Translational Science.2021; 4(2): 858. CrossRef - Antioxidative Potentials of Incretin-Based Medications: A Review of Molecular Mechanisms
Habib Yaribeygi, Mina Maleki, Thozhukat Sathyapalan, Tannaz Jamialahmadi, Amirhossein Sahebkar, Marina Sokovi
Oxidative Medicine and Cellular Longevity.2021; 2021: 1. CrossRef - Vernicia fordii (Hemsl.) Airy Shaw extract stimulates insulin secretion in pancreatic β-cells and improves insulin sensitivity in diabetic mice
Jimin Hyun, Mi Hyeon Park, Yo Han Lee, Youngeun Lee, Su Ji Jeong, Sun Sil Choi, Keon Woo Khim, Hye Jin Eom, Jin-Hoe Hur, Chan Young Park, Jae-Ick Kim, Jiyoung Park, Hyung Won Ryu, Hyun-Jun Jang, Sei-Ryang Oh, Jang Hyun Choi
Journal of Ethnopharmacology.2021; 278: 114238. CrossRef - Connecting the dots between mitochondrial dysfunction and Parkinson’s disorder: focus mitochondria-targeting therapeutic paradigm in mitigating the disease severity
Ishnoor Kaur, Tapan Behl, Aayush Sehgal, Sukhbir Singh, Neelam Sharma, Lotfi Aleya, Simona Bungau
Environmental Science and Pollution Research.2021; 28(28): 37060. CrossRef - Neuromodulatory effects of anti-diabetes medications: A mechanistic review
Habib Yaribeygi, Milad Ashrafizadeh, Neil C. Henney, Thozhukat Sathyapalan, Tannaz Jamialahmadi, Amirhossein Sahebkar
Pharmacological Research.2020; 152: 104611. CrossRef - Antidiabetic Agents for Treatment of Parkinson’s Disease: A Meta-Analysis
Shu-Yi Wang, Shey-Lin Wu, Ta-Cheng Chen, Chieh-Sen Chuang
International Journal of Environmental Research and Public Health.2020; 17(13): 4805. CrossRef - Visualizing subcellular rearrangements in intact β cells using soft x-ray tomography
Kate L. White, Jitin Singla, Valentina Loconte, Jian-Hua Chen, Axel Ekman, Liping Sun, Xianjun Zhang, John Paul Francis, Angdi Li, Wen Lin, Kaylee Tseng, Gerry McDermott, Frank Alber, Andrej Sali, Carolyn Larabell, Raymond C. Stevens
Science Advances.2020;[Epub] CrossRef - Nutrient Sensor mTOR and OGT: Orchestrators of Organelle Homeostasis in Pancreatic β-Cells
Nicholas Esch, Seokwon Jo, Mackenzie Moore, Emilyn U. Alejandro, Yingke Xu
Journal of Diabetes Research.2020; 2020: 1. CrossRef - Metabolic dynamics of human Sertoli cells are differentially modulated by physiological and pharmacological concentrations of GLP-1
Ana D. Martins, Mariana P. Monteiro, Branca M. Silva, Alberto Barros, Mário Sousa, Rui A. Carvalho, Pedro F. Oliveira, Marco G. Alves
Toxicology and Applied Pharmacology.2019; 362: 1. CrossRef - Sarcopenia in Chronic Kidney Disease: Factors, Mechanisms, and Therapeutic Interventions
Hiroshi Watanabe, Yuki Enoki, Toru Maruyama
Biological and Pharmaceutical Bulletin.2019; 42(9): 1437. CrossRef - Recent Advances in Drug Repurposing for Parkinson’s Disease
Xin Chen, Giuseppe Gumina, Kristopher G. Virga
Current Medicinal Chemistry.2019; 26(28): 5340. CrossRef - Mitochondrial dysfunction in diabetes and the regulatory roles of antidiabetic agents on the mitochondrial function
Habib Yaribeygi, Stephen L. Atkin, Amirhossein Sahebkar
Journal of Cellular Physiology.2019; 234(6): 8402. CrossRef - Liraglutide protects against glucolipotoxicity‐induced RIN‐m5F β‐cell apoptosis through restoration of PDX1 expression
Edy Kornelius, Hsin‐Hua Li, Chiung‐Huei Peng, Yi‐Sun Yang, Wei‐Jen Chen, Yan‐Zin Chang, Yi‐Chiao Bai, Stanley Liu, Chien‐Ning Huang, Chih‐Li Lin
Journal of Cellular and Molecular Medicine.2019; 23(1): 619. CrossRef - The osteogenic effect of liraglutide involves enhanced mitochondrial biogenesis in osteoblasts
Subhashis Pal, Shailendra K. Maurya, Sourav Chattopadhyay, Shyamsundar Pal China, Konica Porwal, Chirag Kulkarni, Sabyasachi Sanyal, Rohit A. Sinha, Naibedya Chattopadhyay
Biochemical Pharmacology.2019; 164: 34. CrossRef - Novel Treatment Opportunities Against Cognitive Impairment in Parkinson’s Disease with an Emphasis on Diabetes-Related Pathways
Holly Green, Panagiota Tsitsi, Ioanna Markaki, Dag Aarsland, Per Svenningsson
CNS Drugs.2019; 33(2): 143. CrossRef - Mitral cells and the glucagon‐like peptide 1 receptor: The sweet smell of success?
Enrico Bagnoli, Una FitzGerald
European Journal of Neuroscience.2019; 49(4): 422. CrossRef - Targeting the DPP-4-GLP-1 pathway improves exercise tolerance in heart failure patients: a systematic review and meta-analysis
Chengcong Chen, Ying Huang, Yongmei Zeng, Xiyan Lu, Guoqing Dong
BMC Cardiovascular Disorders.2019;[Epub] CrossRef - Acute exposure to 3‑deoxyglucosone at high glucose levels impairs insulin secretion from β‑cells by downregulating the sweet taste receptor signaling pathway
Xiudao Song, Guoqiang Liang, Min Shi, Liang Zhou, Fei Wang, Lurong Zhang, Fei Huang, Guorong Jiang
Molecular Medicine Reports.2019;[Epub] CrossRef - Translational approaches to restoring mitochondrial function in Parkinson's disease
Heather Mortiboys, Ruby Macdonald, Thomas Payne, Matilde Sassani, Thomas Jenkins, Oliver Bandmann
FEBS Letters.2018; 592(5): 776. CrossRef - Neuroprotective exendin-4 enhances hypothermia therapy in a model of hypoxic-ischaemic encephalopathy
Eridan Rocha-Ferreira, Laura Poupon, Aura Zelco, Anna-Lena Leverin, Syam Nair, Andrea Jonsdotter, Ylva Carlsson, Claire Thornton, Henrik Hagberg, Ahad A Rahim
Brain.2018; 141(10): 2925. CrossRef - Current perspective of mitochondrial biology in Parkinson's disease
Navneet Ammal Kaidery, Bobby Thomas
Neurochemistry International.2018; 117: 91. CrossRef - Humanin promotes mitochondrial biogenesis in pancreatic MIN6 β-cells
Qingqing Qin, Jieqiong Jin, Fang He, Yongqin Zheng, Tingting Li, Yun Zhang, Jundong He
Biochemical and Biophysical Research Communications.2018; 497(1): 292. CrossRef - Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial
Dilan Athauda, Kate Maclagan, Simon S Skene, Martha Bajwa-Joseph, Dawn Letchford, Kashfia Chowdhury, Steve Hibbert, Natalia Budnik, Luca Zampedri, John Dickson, Yazhou Li, Iciar Aviles-Olmos, Thomas T Warner, Patricia Limousin, Andrew J Lees, Nigel H Grei
The Lancet.2017; 390(10103): 1664. CrossRef - Alogliptin, a Dipeptidyl Peptidase‐4 Inhibitor, Alleviates Atrial Remodeling and Improves Mitochondrial Function and Biogenesis in Diabetic Rabbits
Xiaowei Zhang, Zhiwei Zhang, Yungang Zhao, Ning Jiang, Jiuchun Qiu, Yajuan Yang, Jian Li, Xue Liang, Xinghua Wang, Gary Tse, Guangping Li, Tong Liu
Journal of the American Heart Association.2017;[Epub] CrossRef - Potential therapeutic interventions for chronic kidney disease‐associated sarcopenia via indoxyl sulfate‐induced mitochondrial dysfunction
Yuki Enoki, Hiroshi Watanabe, Riho Arake, Rui Fujimura, Kana Ishiodori, Tadashi Imafuku, Kento Nishida, Ryusei Sugimoto, Saori Nagao, Shigeyuki Miyamura, Yu Ishima, Motoko Tanaka, Kazutaka Matsushita, Hirotaka Komaba, Masafumi Fukagawa, Masaki Otagiri, To
Journal of Cachexia, Sarcopenia and Muscle.2017; 8(5): 735. CrossRef - The pancreas: Bandmaster of glucose homeostasis
Nathalie Jouvet, Jennifer L. Estall
Experimental Cell Research.2017; 360(1): 19. CrossRef - The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: mechanisms of action
Dilan Athauda, Thomas Foltynie
Drug Discovery Today.2016; 21(5): 802. CrossRef - Effects of addition of a dipeptidyl peptidase IV inhibitor to metformin on sirolimus-induced diabetes mellitus
Long Jin, Sun Woo Lim, Jian Jin, Byung Ha Chung, Chul Woo Yang
Translational Research.2016; 174: 122. CrossRef - Pharmacological Modulators of Endoplasmic Reticulum Stress in Metabolic Diseases
Tae Jung, Kyung Choi
International Journal of Molecular Sciences.2016; 17(2): 192. CrossRef
- New fraternine analogues: Evaluation of the antiparkinsonian effect in the model of Parkinson's disease

- Thyroid
- Mitochondrial Energy Metabolism and Thyroid Cancers
- Junguee Lee, Joon Young Chang, Yea Eun Kang, Shinae Yi, Min Hee Lee, Kyong Hye Joung, Kun Soon Kim, Minho Shong
- Endocrinol Metab. 2015;30(2):117-123. Published online June 30, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.2.117
- 4,308 View
- 50 Download
- 15 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Primary thyroid cancers including papillary, follicular, poorly differentiated, and anaplastic carcinomas show substantial differences in biological and clinical behaviors. Even in the same pathological type, there is wide variability in the clinical course of disease progression. The molecular carcinogenesis of thyroid cancer has advanced tremendously in the last decade. However, specific inhibition of oncogenic pathways did not provide a significant survival benefit in advanced progressive thyroid cancer that is resistant to radioactive iodine therapy. Accumulating evidence clearly shows that cellular energy metabolism, which is controlled by oncogenes and other tumor-related factors, is a critical factor determining the clinical phenotypes of cancer. However, the role and nature of energy metabolism in thyroid cancer remain unclear. In this article, we discuss the role of cellular energy metabolism, particularly mitochondrial energy metabolism, in thyroid cancer. Determining the molecular nature of metabolic remodeling in thyroid cancer may provide new biomarkers and therapeutic targets that may be useful in the management of refractory thyroid cancers.
-
Citations
Citations to this article as recorded by- Exploring the clinical utility of DPP-IV and SGLT2 inhibitors in papillary thyroid cancer: a literature review
Angelika Buczyńska, Maria Kościuszko, Adam Jacek Krętowski, Anna Popławska-Kita
Frontiers in Pharmacology.2024;[Epub] CrossRef - Liquid Biopsy as a Method for Minimally Invasive Diagnosis of Thyroid Cancer
Tagir I. Rakhmatullin, Mark Jain, Larisa M. Samokhodskaya, Vladimir A. Zhivotov
Journal of Clinical Practice.2023; 14(3): 69. CrossRef - Development of Metabolic Synthetic Lethality and Its Implications for Thyroid Cancer
Sang-Hyeon Ju, Seong Eun Lee, Yea Eun Kang, Minho Shong
Endocrinology and Metabolism.2022; 37(1): 53. CrossRef - Monensin Inhibits Anaplastic Thyroid Cancer via Disrupting Mitochondrial Respiration
and AMPK/mTOR Signaling
Yanli Li, Qianshu Sun, Sisi Chen, Xiongjie Yu, Hongxia Jing
Anti-Cancer Agents in Medicinal Chemistry.2022; 22(14): 2539. CrossRef - Growth Differentiation Factor 15 is a Cancer Cell-Induced Mitokine That Primes Thyroid Cancer Cells for Invasiveness
Yea Eun Kang, Jin Man Kim, Mi Ae Lim, Seong Eun Lee, Shinae Yi, Jung Tae Kim, Chan Oh, Lihua Liu, Yanli Jin, Seung-Nam Jung, Ho-Ryun Won, Jae Won Chang, Jeong Ho Lee, Hyun Jung Kim, Hyun Yong Koh, Sangmi Jun, Sun Wook Cho, Minho Shong, Bon Seok Koo
Thyroid.2021; 31(5): 772. CrossRef - Clinical Significance of the D-Loop Gene Mutation in Mitochondrial DNA in Laryngeal Cancer
Lei Wang, He-Xiang Cheng, Yan-Hui Zhou, Min Ma
OncoTargets and Therapy.2021; Volume 14: 3461. CrossRef - Transcriptomic and Genetic Associations between Alzheimer’s Disease, Parkinson’s Disease, and Cancer
Jaume Forés-Martos, Cesar Boullosa, David Rodrigo-Domínguez, Jon Sánchez-Valle, Beatriz Suay-García, Joan Climent, Antonio Falcó, Alfonso Valencia, Joan Anton Puig-Butillé, Susana Puig, Rafael Tabarés-Seisdedos
Cancers.2021; 13(12): 2990. CrossRef - KLF5 influences cell biological function and chemotherapy sensitivity through the JNK signaling pathway in anaplastic thyroid carcinoma
Zheng Wang, Xinguang Qiu, Hao Zhang, Weihan Li
Journal of Biochemical and Molecular Toxicology.2020;[Epub] CrossRef - Metabolic reprogramming related to whole-chromosome instability in models for Hürthle cell carcinoma
Ruben D. Addie, Sarantos Kostidis, Willem E. Corver, Jan Oosting, Sepideh Aminzadeh-Gohari, René G. Feichtinger, Barbara Kofler, Mehtap Derya Aydemirli, Martin Giera, Hans Morreau
Scientific Reports.2020;[Epub] CrossRef - Inhibition of mitochondrial respiration by tigecycline selectively targets thyroid carcinoma and increases chemosensitivity
Yuehua Wang, Fei Xie, Dejie Chen, Ling Wang
Clinical and Experimental Pharmacology and Physiology.2019; 46(10): 890. CrossRef - Investigating Therapeutic Effects of Retinoic Acid on Thyroid Cancer via Protein-Protein Interaction Network Analysis
Majid Rezaei-Tavirani, Mostafa Rezaei-Tavirani, Mona Zamanian Azodi
International Journal of Cancer Management.2019;[Epub] CrossRef - CASE REPORT: An Extensively Necrotic Hürthle-Cell Carcinoma Mimicked a Thyroid Abscess
Sanders H. Lin, Shih-Ming Huang, Su-Lin Peng
Clinical Thyroidology.2018; 30(11): 529. CrossRef - Atovaquone enhances doxorubicin’s efficacy via inhibiting mitochondrial respiration and STAT3 in aggressive thyroid cancer
Zhuo Lv, Xintong Yan, Liying Lu, Chun Su, Yin He
Journal of Bioenergetics and Biomembranes.2018; 50(4): 263. CrossRef - Identification of novel biomarker and therapeutic target candidates for diagnosis and treatment of follicular carcinoma
Xianyin Lai, Christopher B. Umbricht, Kurt Fisher, Justin Bishop, Qiuying Shi, Shaoxiong Chen
Journal of Proteomics.2017; 166: 59. CrossRef - Pathological processes and therapeutic advances in radioiodide refractory thyroid cancer
Marika H Tesselaar, Johannes W Smit, James Nagarajah, Romana T Netea-Maier, Theo S Plantinga
Journal of Molecular Endocrinology.2017; 59(4): R141. CrossRef - Integrated microRNA, gene expression and transcription factors signature in papillary thyroid cancer with lymph node metastasis
Nurul-Syakima Ab Mutalib, Sri Noraima Othman, Azliana Mohamad Yusof, Shahrun Niza Abdullah Suhaimi, Rohaizak Muhammad, Rahman Jamal
PeerJ.2016; 4: e2119. CrossRef
- Exploring the clinical utility of DPP-IV and SGLT2 inhibitors in papillary thyroid cancer: a literature review

- Obesity and Metabolism
- Mitochondrial Complexes I and II Are More Susceptible to Autophagy Deficiency in Mouse β-Cells
- Min Joo Kim, Ok Kyong Choi, Kyung Sil Chae, Min Kyeong Kim, Jung Hee Kim, Masaaki Komatsu, Keiji Tanaka, Hakmo Lee, Sung Soo Chung, Soo Heon Kwak, Young Min Cho, Kyong Soo Park, Hye Seung Jung
- Endocrinol Metab. 2015;30(1):65-70. Published online March 27, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.1.65
- 3,939 View
- 40 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Damaged mitochondria are removed by autophagy. Therefore, impairment of autophagy induces the accumulation of damaged mitochondria and mitochondrial dysfunction in most mammalian cells. Here, we investigated mitochondrial function and the expression of mitochondrial complexes in autophagy-related 7 (
Atg7 )-deficient β-cells.Methods To evaluate the effect of autophagy deficiency on mitochondrial function in pancreatic β-cells, we isolated islets from
Atg7 F/F:RIP-Cre + mice and wild-type littermates. Oxygen consumption rate and intracellular adenosine 5'-triphosphate (ATP) content were measured. The expression of mitochondrial complex genes inAtg7 -deficient islets and in β-TC6 cells transfected with siAtg7 was measured by quantitative real-time polymerase chain reaction.Results Baseline oxygen consumption rate of
Atg7 -deficient islets was significantly lower than that of control islets (P <0.05). Intracellular ATP content ofAtg7 -deficient islets during glucose stimulation was also significantly lower than that of control islets (P <0.05). By Oxygraph-2k analysis, mitochondrial respiration inAtg7 -deficient islets was significantly decreased overall, although state 3 respiration and responses to antimycin A were unaffected. The mRNA levels of mitochondrial complexes I, II, III, and V inAtg7 -deficient islets were significantly lower than in control islets (P <0.05). Down-regulation ofAtg7 in β-TC6 cells also reduced the expression of complexes I and II, with marginal significance (P <0.1).Conclusion Impairment of autophagy in pancreatic β-cells suppressed the expression of some mitochondrial respiratory complexes, and may contribute to mitochondrial dysfunction. Among the complexes, I and II seem to be most vulnerable to autophagy deficiency.
-
Citations
Citations to this article as recorded by- Proteomic pathways to metabolic disease and type 2 diabetes in the pancreatic islet
Belinda Yau, Sheyda Naghiloo, Alexis Diaz-Vegas, Austin V. Carr, Julian Van Gerwen, Elise J. Needham, Dillon Jevon, Sing-Young Chen, Kyle L. Hoehn, Amanda E. Brandon, Laurence Macia, Gregory J. Cooney, Michael R. Shortreed, Lloyd M. Smith, Mark P. Keller,
iScience.2021; 24(10): 103099. CrossRef - Natural compound oblongifolin C inhibits autophagic flux, and induces apoptosis and mitochondrial dysfunction in human cholangiocarcinoma QBC939 cells
Aiqing Zhang, Wei He, Huimin Shi, Xiaodan Huang, Guozhong Ji
Molecular Medicine Reports.2016; 14(4): 3179. CrossRef - Autophagy deficiency in β cells blunts incretin-induced suppression of glucagon release from α cells
Min Joo Kim, Ok Kyong Choi, Kyung Sil Chae, Hakmo Lee, Sung Soo Chung, Dong-Sik Ham, Ji-Won Kim, Kun-Ho Yoon, Kyong Soo Park, Hye Seung Jung
Islets.2015; 7(5): e1129096. CrossRef
- Proteomic pathways to metabolic disease and type 2 diabetes in the pancreatic islet


 KES
KES

 First
First Prev
Prev



