Search
- Page Path
- HOME > Search
Original Article
- Miscellaneous
- Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
- Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
- Endocrinol Metab. 2023;38(6):750-759. Published online November 13, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1785
- 1,432 View
- 120 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the incidence of endocrine immune-related adverse events (irAEs) for recently developed immune checkpoint inhibitor (ICI) drugs.
Methods
We collected studies on newly developed ICI drugs using PubMed/Medline, Embase, and Cochrane Library from inception through January 31, 2023. Among ICI drugs, nivolumab, pembrolizumab, and ipilimumab were excluded from the new ICI drugs because many papers on endocrine-related side effects have already been published.
Results
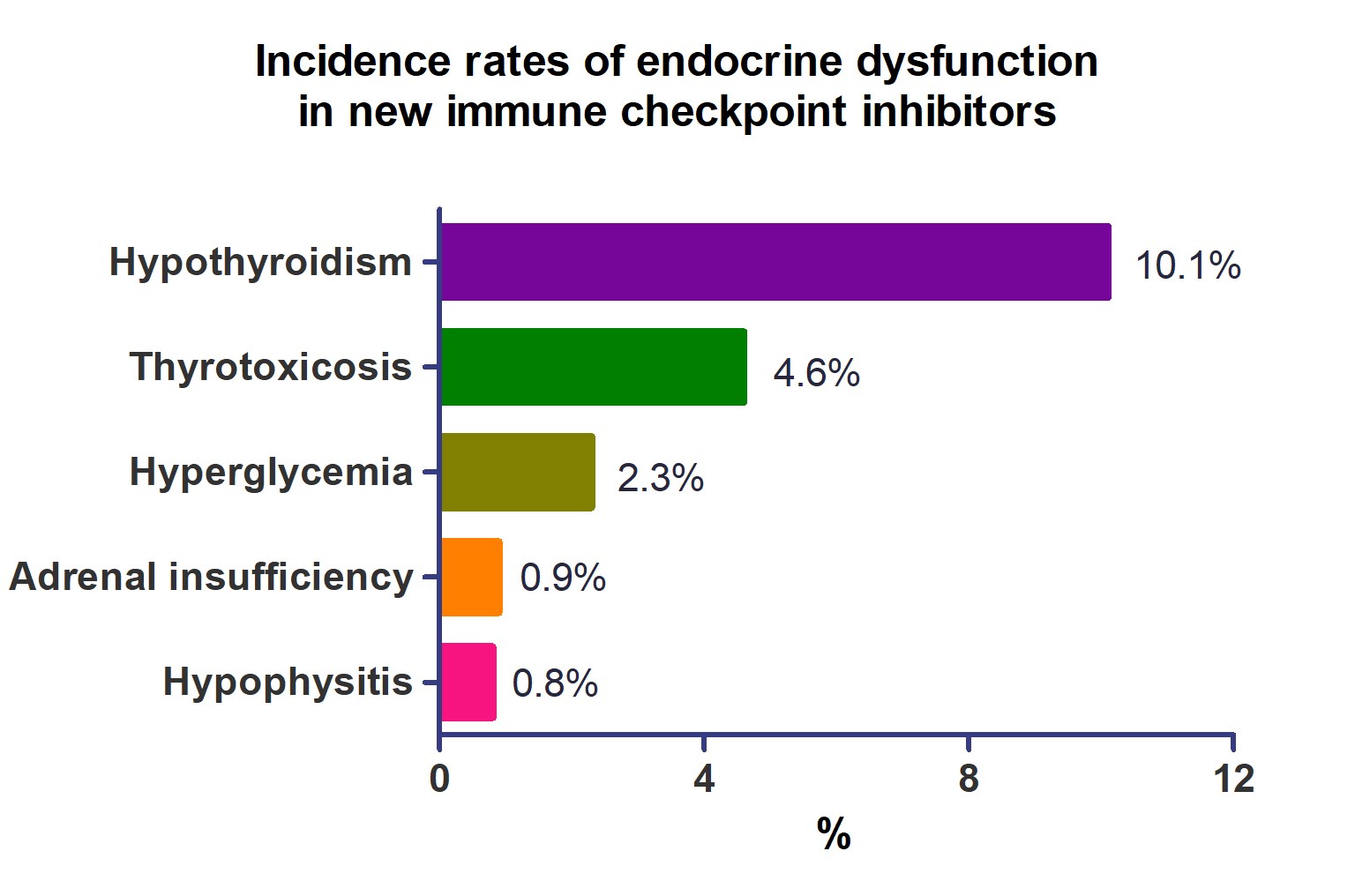
A total of 44,595 patients from 177 studies were included in this analysis. The incidence of hypothyroidism was 10.1% (95% confidence interval [CI], 8.9% to 11.4%), thyrotoxicosis was 4.6% (95% CI, 3.8% to 5.7%), hypophysitis was 0.8% (95% CI, 0.5% to 1.1%), adrenal insufficiency was 0.9% (95% CI, 0.7% to 1.1%), and hyperglycemia was 2.3% (95% CI, 1.6% to 3.4%). Hypothyroidism and thyrotoxicosis occurred most frequently with programmed cell death protein-1 (PD-1) inhibitors (13.7% and 7.5%, respectively). The rate of endocrine side effects for the combination of a programmed death-ligand 1 inhibitor (durvalumab) and cytotoxic T lymphocyte-associated antigen 4 inhibitor (tremelimumab) was higher than that of monotherapy. In a meta-analysis, the combination of tremelimumab and durvalumab had a 9- to 10-fold higher risk of pituitary and adrenal-related side effects than durvalumab alone.
Conclusion
Newly developed PD-1 inhibitors had a high incidence of thyroid-related irAEs, and combined treatment with durvalumab and tremelimumab increased the risk of pituitary- and adrenal-related irAEs. Based on these facts, it is necessary to predict the endocrine side effects corresponding to each ICI drug, diagnose and treat them appropriately, and try to reduce the morbidity and mortality of patients.

Special Article
- Miscellaneous
- Immune Checkpoint Inhibitors and Endocrine Disorders: A Position Statement from the Korean Endocrine Society
- Hyemi Kwon, Eun Roh, Chang Ho Ahn, Hee Kyung Kim, Cheol Ryong Ku, Kyong Yeun Jung, Ju Hee Lee, Eun Heui Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Jun Sung Moon, Jin Hwa Kim, Mi-kyung Kim, The Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2022;37(6):839-850. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1627
- 3,474 View
- 320 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
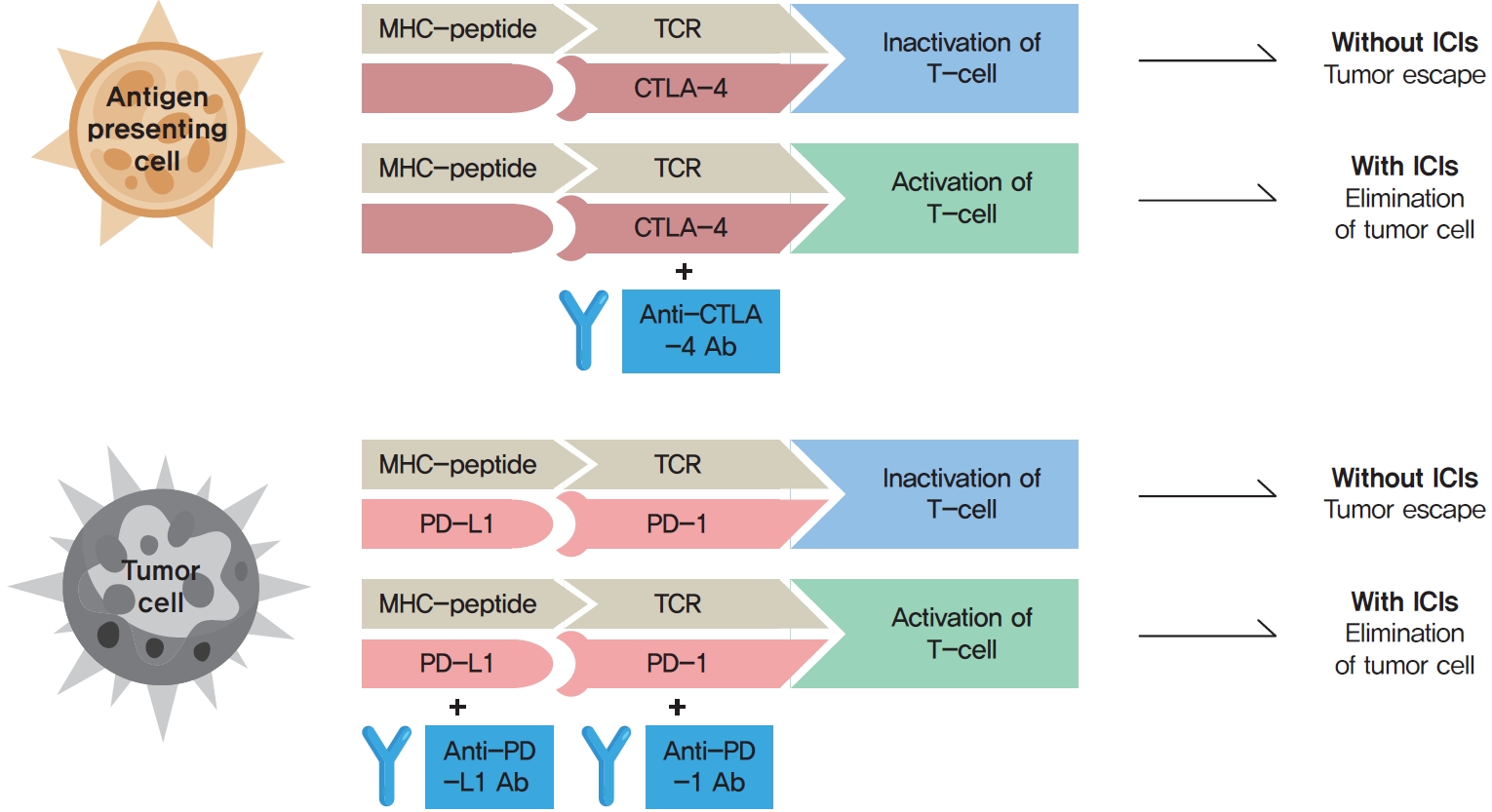
ePub - Immune checkpoint inhibitors (ICIs) including an anti-cytotoxic T-lymphocyte-associated antigen 4 inhibitor, anti-programmed cell death protein 1 (PD-1) inhibitors, and anti-PD-ligand 1 inhibitors are representative therapeutics for various malignancies. In oncology, the application of ICIs is currently expanding to a wider range of malignancies due to their remarkable clinical outcomes. ICIs target immune checkpoints which suppress the activity of T-cells that are specific for tumor antigens, thereby allowing tumor cells to escape the immune response. However, immune checkpoints also play a crucial role in preventing autoimmune reactions. Therefore, ICIs targeting immune checkpoints can trigger various immune-related adverse events (irAEs), especially in endocrine organs. Considering the endocrine organs that are frequently involved, irAEs associated endocrinopathies are frequently life-threatening and have unfavorable clinical implications for patients. However, there are very limited data from large clinical trials that would inform the development of clinical guidelines for patients with irAEs associated endocrinopathies. Considering the current clinical situation, in which the scope and scale of the application of ICIs are increasing, position statements from clinical specialists play an essential role in providing the appropriate recommendations based on both medical evidence and clinical experience. As endocrinologists, we would like to present precautions and recommendations for the management of immune-related endocrine disorders, especially those involving the adrenal, thyroid, and pituitary glands caused by ICIs.
-
Citations
Citations to this article as recorded by- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population
Ryo Fujiwara, Takeshi yuasa, kenichi kobayashi, tetsuya yoshida, susumu kageyama
Expert Review of Anticancer Therapy.2023; 23(5): 461. CrossRef - Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
Endocrinology and Metabolism.2023; 38(6): 750. CrossRef
- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population

Original Article
- Diabetes, Obesity and Metabolism
- Human Leukocyte Antigens and Biomarkers in Type 1 Diabetes Mellitus Induced by Immune-Checkpoint Inhibitors
- Hidefumi Inaba, Yosuke Kaido, Saya Ito, Tomonao Hirobata, Gen Inoue, Takakazu Sugita, Yuki Yamamoto, Masatoshi Jinnin, Hiroaki Kimura, Tomoko Kobayashi, Shintaro Iwama, Hiroshi Arima, Takaaki Matsuoka
- Endocrinol Metab. 2022;37(1):84-95. Published online February 28, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1282
- 4,200 View
- 158 Download
- 16 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Type 1 diabetes mellitus induced by immune-checkpoint inhibitors (ICI-T1DM) is a rare critical entity. However, the etiology of ICI-T1DM remains unclear.
Methods
In order to elucidate risk factors for ICI-T1DM, we evaluated the clinical course and immunological status of patients with ICI-T1DM who had been diagnosed during 2016 to 2021.
Results
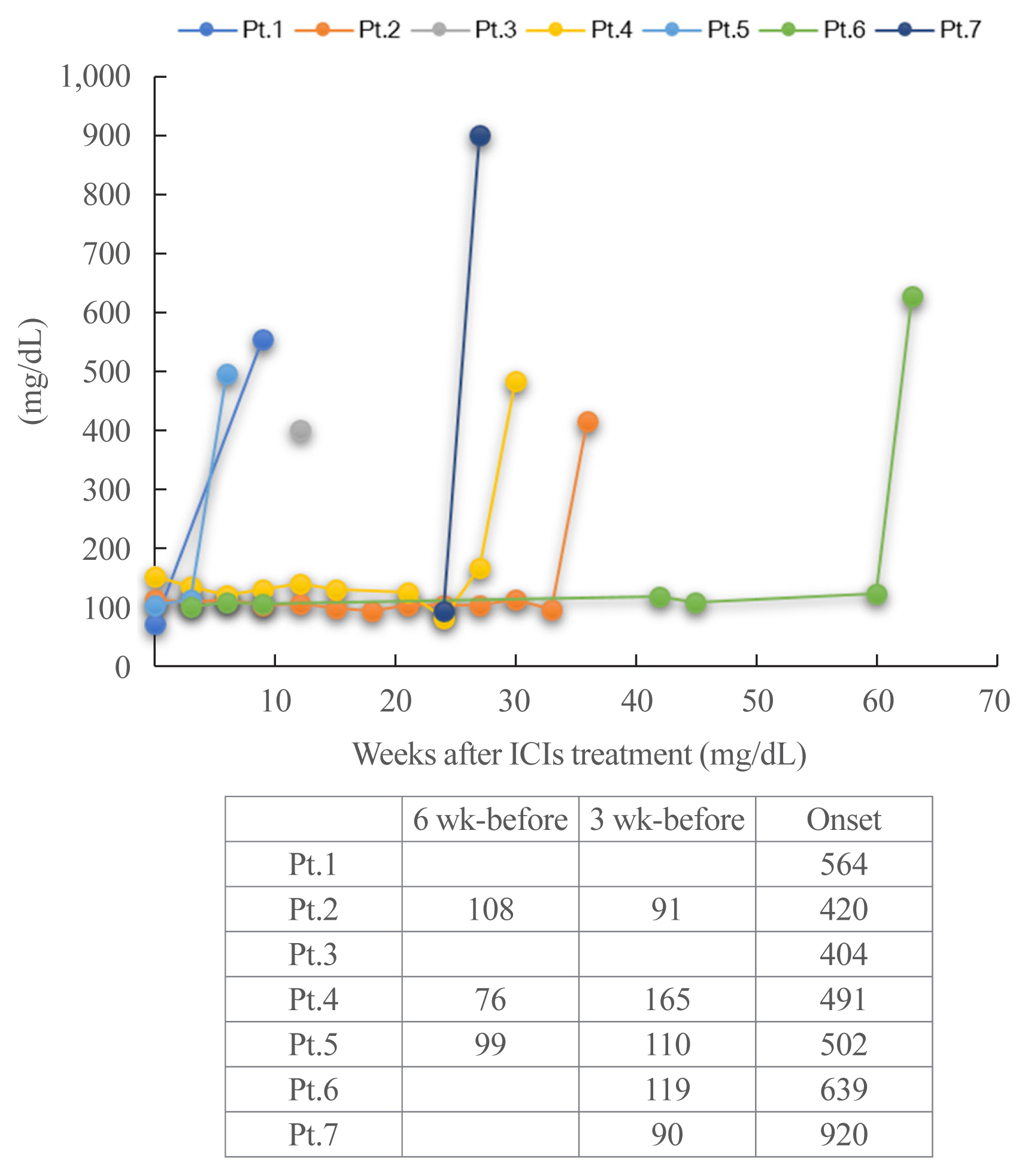
Seven of 871 (0.8%, six men and one woman) patients developed ICI-T1DM. We revealed that the allele frequencies of human leukocyte antigen (HLA)-DPA1*02:02 and DPB1*05:01 were significantly higher in the patients with ICI-T1DM In comparison to the controls who received ICI (11/14 vs. 10/26, P=0.022; 11/14 vs. 7/26, P=0.0027, respectively). HLA-DRB1*04:05, which has been found to be a T1DM susceptibility allele in Asians, was also observed as a high-risk allele for ICI-T1DM. The significance of the HLA-DPB1*05:01 and DRB1*04:05 alleles was confirmed by an analysis of four additional patients. The absolute/relative neutrophil count, neutrophils-lymphocyte ratio, and neutrophil-eosinophil ratio increased, and the absolute lymphocyte count and absolute/relative eosinophil count decreased at the onset as compared with 6 weeks before. In two patients, alterations in cytokines and chemokines were found at the onset.
Conclusion
Novel high-risk HLA alleles and haplotypes were identified in ICI-T1DM, and peripheral blood factors may be utilized as biomarkers. -
Citations
Citations to this article as recorded by- Type 1 diabetes mellitus affected by potential toxicity from long-term use of nivolumab
Yuma Motomura, Shin Urai, Yushi Hirota, Naoki Takegawa, Hironori Bando, Masaaki Yamamoto, Hidenori Fukuoka, Masahiro Tsuda, Wataru Ogawa
Diabetology International.2024; 15(1): 130. CrossRef - Review – The impact of pharmacogenetics on the outcome of immune checkpoint inhibitors
Karlijn de Joode, Niels Heersche, Edwin A. Basak, Sander Bins, Astrid A.M. van der Veldt, Ron H.N. van Schaik, Ron H.J. Mathijssen
Cancer Treatment Reviews.2024; 122: 102662. CrossRef - Reaching the Diagnosis of Checkpoint Inhibitor-Induced Diabetes Mellitus in Different Clinical Scenarios: A Real-World Application of Updated Diagnostic Criteria
Anna Angelousi, Dimitrios C. Ziogas, Vasiliki Siampanopoulou, Chrysoula Mytareli, Amalia Anastasopoulou, George Lyrarakis, Helen Gogas
Diseases.2024; 12(2): 40. CrossRef - Non-Invasive Predictive Biomarkers for Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors
Ben Ponvilawan, Abdul Wali Khan, Janakiraman Subramanian, Dhruv Bansal
Cancers.2024; 16(6): 1225. CrossRef - A case of rapidly progressive insulin-dependent diabetes mellitus without islet autoantibodies developed over two years after the first dose of nivolumab
Kota Nishihama, Yuko Okano, Chisa Inoue, Kanako Maki, Kazuhito Eguchi, Soichiro Tanaka, Atsuro Takeshita, Mei Uemura, Taro Yasuma, Toshinari Suzuki, Esteban C. Gabazza, Yutaka Yano
Diabetology International.2024;[Epub] CrossRef - A single center case series of immune checkpoint inhibitor-induced type 1 diabetes mellitus, patterns of disease onset and long-term clinical outcome
John Marsiglio, Jordan P. McPherson, Magdalena Kovacsovics-Bankowski, Joanne Jeter, Christos Vaklavas, Umang Swami, Douglas Grossmann, Alyssa Erickson-Wayman, Heloisa P. Soares, Katie Kerrigan, Berit Gibson, Jennifer Anne Doherty, John Hyngstrom, Sheetal
Frontiers in Immunology.2023;[Epub] CrossRef - Predictive Biomarkers for Immune-Related Endocrinopathies following Immune Checkpoint Inhibitors Treatment
Almog Shalit, Panagiotis Sarantis, Evangelos Koustas, Eleni-Myrto Trifylli, Dimitris Matthaios, Michalis V. Karamouzis
Cancers.2023; 15(2): 375. CrossRef - Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events
Iñigo Les, Mireia Martínez, Inés Pérez-Francisco, María Cabero, Lucía Teijeira, Virginia Arrazubi, Nuria Torrego, Ana Campillo-Calatayud, Iñaki Elejalde, Grazyna Kochan, David Escors
Cancers.2023; 15(5): 1629. CrossRef - Amino acid polymorphisms in human histocompatibility leukocyte antigen class II and proinsulin epitope have impacts on type 1 diabetes mellitus induced by immune-checkpoint inhibitors
Hidefumi Inaba, Shuhei Morita, Daisuke Kosugi, Yuki Asai, Yosuke Kaido, Saya Ito, Tomonao Hirobata, Gen Inoue, Yuki Yamamoto, Masatoshi Jinnin, Hiroaki Kimura, Masao Ota, Yuko Okudaira, Hiroyasu Nakatani, Tomoko Kobayashi, Shintaro Iwama, Hiroshi Arima, T
Frontiers in Immunology.2023;[Epub] CrossRef - Clinical characteristics and human leukocyte antigens in patients with immune checkpoint inhibitor-induced type 1 diabetes and pituitary dysfunction: a single center prospective study
Natsuko Hara, Hirotsugu Suwanai, Fumiyoshi Yakou, Keitaro Ishii, Hajime Iwasaki, Hironori Abe, Jumpei Shikuma, Hiroyuki Sakai, Takashi Miwa, Ryo Suzuki
Endocrine.2023; 81(3): 477. CrossRef - Autoimmunity in immune checkpoint inhibitor‐induced immune‐related adverse events: A focus on autoimmune skin toxicity and pneumonitis
Fiamma Berner, Lukas Flatz
Immunological Reviews.2023; 318(1): 37. CrossRef - Prediction-based prompt levothyroxine replacement to prevent a hypothyroid state after immune-related adverse events involving the thyroid gland
Ichiro Yamauchi, Takuro Hakata, Taku Sugawa, Daisuke Kosugi, Haruka Fujita, Kentaro Okamoto, Yohei Ueda, Toshihito Fujii, Daisuke Taura, Norio Harada, Nobuya Inagaki
Endocrine Journal.2023; 70(10): 987. CrossRef - Key Determinants of Immune-Mediated Adverse Reactions to Oncology Drugs
Yihan Zhou, Shan Ding
Cancers.2023; 15(23): 5622. CrossRef - Risk factors and predictors of immune-related adverse events: implications for patients with non-small cell lung cancer
Majd Issa, Joy Tang, Yizhen Guo, Chris Coss, Thomas A. Mace, Jason Bischof, Mitch Phelps, Carolyn J Presley, Dwight H Owen
Expert Review of Anticancer Therapy.2022; 22(8): 861. CrossRef - Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors
Adithya Chennamadhavuni, Laith Abushahin, Ning Jin, Carolyn J. Presley, Ashish Manne
Frontiers in Immunology.2022;[Epub] CrossRef - Flash Glucose Monitoring and Diabetes Mellitus Induced by Immune Checkpoint Inhibitors: An Approach to Clinical Practice
Pablo Rodríguez de Vera-Gómez, Ana Piñar-Gutiérrez, Raquel Guerrero-Vázquez, Virginia Bellido, Cristóbal Morales-Portillo, María Pilar Sancho-Márquez, Pablo Espejo-García, Noelia Gros-Herguido, Gema López-Gallardo, María Asunción Martínez-Brocca, Alfonso
Journal of Diabetes Research.2022; 2022: 1. CrossRef
- Type 1 diabetes mellitus affected by potential toxicity from long-term use of nivolumab

Review Article
- Miscellaneous
- Clinical Characteristics, Management, and Potential Biomarkers of Endocrine Dysfunction Induced by Immune Checkpoint Inhibitors
- Shintaro Iwama, Tomoko Kobayashi, Hiroshi Arima
- Endocrinol Metab. 2021;36(2):312-321. Published online April 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1007
- 5,461 View
- 266 Download
- 15 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
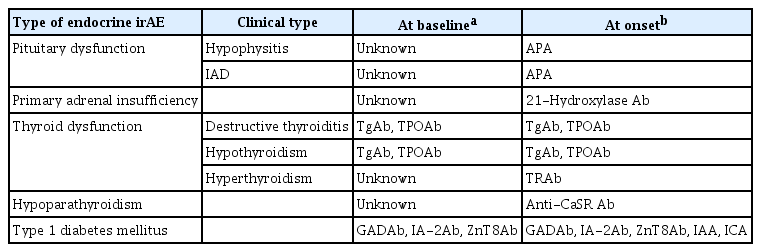
ePub - Immune-related adverse events (irAEs) affecting the endocrine glands are among the most frequent irAEs induced by immune checkpoint inhibitors (ICIs) and include hypopituitarism, primary adrenal insufficiency, thyrotoxicosis, hypothyroidism, hypoparathyroidism, and type 1 diabetes mellitus. Since the incidence and clinical features of endocrine irAEs vary according to the ICI used, it is important to understand the characteristics of these irAEs and to manage each one appropriately. Since some endocrine irAEs, including adrenal crisis and diabetic ketoacidosis, are potentially life-threatening, predicting the risk of endocrine irAEs before their onset is critical. Several autoantibodies have been detected in patients who develop endocrine irAEs, among which anti-thyroid antibodies may be predictive biomarkers of thyroid dysfunction. In this review, we describe the clinical features of each endocrine irAE induced by ICIs and discuss their potential biomarkers, including autoantibodies.
-
Citations
Citations to this article as recorded by- Clinical characteristics and potential biomarkers of thyroid and pituitary immune-related adverse events
Tomoko Kobayashi, Shintaro Iwama, Hiroshi Arima
Endocrine Journal.2024; 71(1): 23. CrossRef - A case of rapidly progressive insulin-dependent diabetes mellitus without islet autoantibodies developed over two years after the first dose of nivolumab
Kota Nishihama, Yuko Okano, Chisa Inoue, Kanako Maki, Kazuhito Eguchi, Soichiro Tanaka, Atsuro Takeshita, Mei Uemura, Taro Yasuma, Toshinari Suzuki, Esteban C. Gabazza, Yutaka Yano
Diabetology International.2024;[Epub] CrossRef - Endocrinopathies Associated With Immune Checkpoint Inhibitor Use
Anupam Kotwal, Randol Kennedy, Nupur Kikani, Sonali Thosani, Whitney Goldner, Afreen Shariff
Endocrine Practice.2024;[Epub] CrossRef - Recovery from insulin dependence in immune checkpoint inhibitor‐associated diabetes mellitus: A case report
Marie Okubo, Yuji Hataya, Kanta Fujimoto, Toshio Iwakura, Naoki Matsuoka
Journal of Diabetes Investigation.2023; 14(1): 147. CrossRef - Case Report: A Rising Cause of New-Onset Endocrinopathies After Immunotherapy
Charity Tan, Sarah Hendricks, Kristina Hernandez, Martha Benavides, Rupinderjit Samra
The Journal for Nurse Practitioners.2023; 19(5): 104582. CrossRef - Risk of Thyroid Dysfunction in PD-1 Blockade Is Stratified by the Pattern of TgAb and TPOAb Positivity at Baseline
Xin Zhou, Shintaro Iwama, Tomoko Kobayashi, Masahiko Ando, Hiroshi Arima
The Journal of Clinical Endocrinology & Metabolism.2023; 108(10): e1056. CrossRef - Severe thyrotoxicosis induced by tislelizumab: a case report and literature review
Liman Huo, Chao Wang, Haixia Ding, Xuelian Shi, Bin Shan, Ruoying Zhou, Ping Liang, Juan Hou
Frontiers in Oncology.2023;[Epub] CrossRef - Life-Threatening Endocrinological Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy
Aleksandra Basek, Grzegorz K. Jakubiak, Grzegorz Cieślar, Agata Stanek
Cancers.2023; 15(24): 5786. CrossRef - Increased Risk of Thyroid Dysfunction by PD-1 and CTLA-4 Blockade in Patients Without Thyroid Autoantibodies at Baseline
Shintaro Iwama, Tomoko Kobayashi, Yoshinori Yasuda, Takayuki Okuji, Masaaki Ito, Masahiko Ando, Xin Zhou, Ayana Yamagami, Takeshi Onoue, Yohei Kawaguchi, Takashi Miyata, Mariko Sugiyama, Hiroshi Takagi, Daisuke Hagiwara, Hidetaka Suga, Ryoichi Banno, Tets
The Journal of Clinical Endocrinology & Metabolism.2022; 107(4): e1620. CrossRef - Biomarkers and risk factors for the early prediction of immune-related adverse events: a review
Ying Zhang, Xiaoling Zhang, Weiling Li, Yunyi Du, Wenqing Hu, Jun Zhao
Human Vaccines & Immunotherapeutics.2022;[Epub] CrossRef - Immune Checkpoint Inhibitors as a Threat to the Hypothalamus–Pituitary Axis: A Completed Puzzle
Agnese Barnabei, Andrea Corsello, Rosa Maria Paragliola, Giovanni Maria Iannantuono, Luca Falzone, Salvatore Maria Corsello, Francesco Torino
Cancers.2022; 14(4): 1057. CrossRef - Elevated TSH Level, TgAb, and Prior Use of Ramucirumab or TKIs as Risk Factors for Thyroid Dysfunction in PD-L1 Blockade
Tomoko Kobayashi, Shintaro Iwama, Ayana Yamagami, Yoshinori Yasuda, Takayuki Okuji, Masaaki Ito, Xin Zhou, Masahiko Ando, Takeshi Onoue, Takashi Miyata, Mariko Sugiyama, Daisuke Hagiwara, Hidetaka Suga, Ryoichi Banno, Tetsunari Hase, Masahiro Morise, Taka
The Journal of Clinical Endocrinology & Metabolism.2022; 107(10): e4115. CrossRef - Preconditioning of the immune system modulates the response of papillary thyroid cancer to immune checkpoint inhibitors
Fabiana Pani, Yoshinori Yasuda, Sylvie T Rousseau, Kevin C Bermea, Solmaz Roshanmehr, Rulin Wang, Srinivasan Yegnasubramanian, Patrizio Caturegli, Luigi Adamo
Journal for ImmunoTherapy of Cancer.2022; 10(12): e005538. CrossRef - Survival benefit of endocrine dysfunction following immune checkpoint inhibitors for nonthyroidal cancers
Anupam Kotwal, Mabel Ryder
Current Opinion in Endocrinology, Diabetes & Obesity.2021; 28(5): 517. CrossRef
- Clinical characteristics and potential biomarkers of thyroid and pituitary immune-related adverse events

Original Article
- Clinical Study
- Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors
- Jee Hee Yoon, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2021;36(2):413-423. Published online April 6, 2021
- DOI: https://doi.org/10.3803/EnM.2020.906
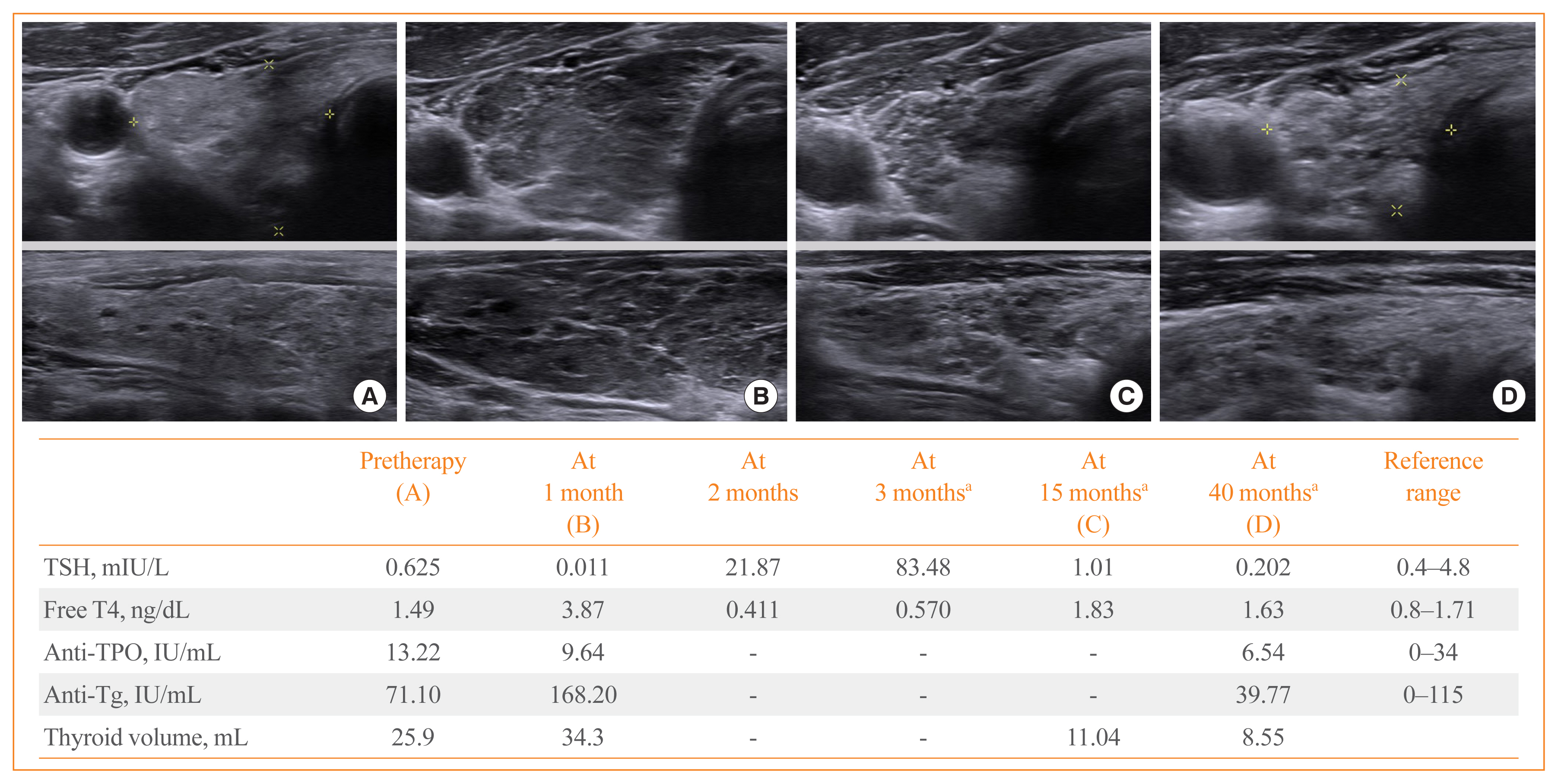
- 5,609 View
- 217 Download
- 23 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Thyroid immune-related adverse events (IRAEs) have been reported in patients treated with programmed cell death protein-1 (PD-1) and programmed cell death protein-ligand 1 (PD-L1) inhibitors. We investigated the incidence and clinical course of PD-1/PD-L1 inhibitor-induced thyroid IRAEs, and identified predictable clinical risk factors of thyroid IRAEs, in particular, overt hypothyroidism (OH).
Methods
We retrospectively reviewed the medical records of 325 cancer patients receiving PD-1/PD-L1 inhibitor in a tertiary referral center.
Results
A total of 50.5% (164/325) of patients experienced at least one abnormal thyroid function following PD-1/PD-L1 inhibitor. Eighty-four patients (51.2%) of them recovered to normal thyroid function during follow-up. In overall population, 25 patients (7.7%) required thyroid hormone replacement therapy due to PD-1/PD-L1 inhibitor-induced OH. Patients who progressed to OH showed significantly higher baseline thyroid stimulating hormone level and longer duration of PD-1/PD-L1 inhibitor therapy than those without thyroid dysfunction or OH (both P<0.001). Median time interval to the development of OH was 3 months after the therapy. OH was significantly associated with positive anti-thyroid peroxidase antibody at baseline and anti-thyroglobulin antibody during the therapy than those without thyroid dysfunction or OH (P=0.015 and P=0.005, respectively). We observed no patients with OH who were able to stop levothyroxine replacement after the cessation of PD-1/PD-L1 inhibitor therapy.
Conclusion
PD-1/PD-L1 inhibitor-induced thyroid dysfunctions are considerably reversible; however, OH is irreversible requiring levothyroxine replacement even after stopping the therapy. Positive thyroid autoantibodies may predict the progression to OH. -
Citations
Citations to this article as recorded by- Thyroid Dysfunction after Atezolizumab and Bevacizumab Is Associated with Favorable Outcomes in Hepatocellular Carcinoma
Young Shin Song, Hannah Yang, Beodeul Kang, Jaekyung Cheon, Ilhwan Kim, Hyeyeong Kim, Won Suk Lee, Yun Beom Sang, Sanghoon Jung, Ho Yeong Lim, Vincent E. Gaillard, Chan Kim, Hong Jae Chon
Liver Cancer.2024; 13(1): 89. CrossRef - Thyroid dysfunction (TD) induced by PD-1/PD-L1 inhibitors in advanced lung cancer
Yanling Wang, Xiaoxuan Yang, Jia Ma, Shenglan Chen, Ping Gong, Ping Dai
Heliyon.2024; 10(5): e27077. CrossRef - Non-Invasive Predictive Biomarkers for Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors
Ben Ponvilawan, Abdul Wali Khan, Janakiraman Subramanian, Dhruv Bansal
Cancers.2024; 16(6): 1225. CrossRef - Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy
David Dora, Syeda Mahak Zahra Bokhari, Kenan Aloss, Peter Takacs, Juliane Zsuzsanna Desnoix, György Szklenárik, Patrick Deniz Hurley, Zoltan Lohinai
International Journal of Molecular Sciences.2023; 24(3): 2769. CrossRef - Immune checkpoint inhibitor-associated toxicity in advanced non-small cell lung cancer: An updated understanding of risk factors
Xiangxiao Hu, Lina Wang, Bin Shang, Junren Wang, Jian Sun, Bin Liang, Lili Su, Wenjie You, Shujuan Jiang
Frontiers in Immunology.2023;[Epub] CrossRef - Immune-related adverse events induced by programmed death protein-1 inhibitors from the perspective of lymphoma immunotherapy
Yong-Zhe Hou, Qin Zhang, Hai Bai, Tao Wu, Ya-Jie Chen
World Journal of Clinical Cases.2023; 11(7): 1458. CrossRef - Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events
Iñigo Les, Mireia Martínez, Inés Pérez-Francisco, María Cabero, Lucía Teijeira, Virginia Arrazubi, Nuria Torrego, Ana Campillo-Calatayud, Iñaki Elejalde, Grazyna Kochan, David Escors
Cancers.2023; 15(5): 1629. CrossRef - Immune-related thyroid dysfunctions during anti PD-1/PD-L1 inhibitors: new evidence from a single centre experience
Alice Nervo, Matteo Ferrari, Giovanni Gruosso, Enrica Migliore, Sara Basile, Valentina D’Angelo, Anna Roux, Alessandro Piovesan, Emanuela Arvat
Clinical and Experimental Medicine.2023; 23(8): 4817. CrossRef - RNA Sequencing Reveals Unique Transcriptomic Signatures of the Thyroid in a Murine Lung Cancer Model Treated with PD-1 and PD-L1 Antibodies
Rena Pollack, Joshua Stokar, Natan Lishinsky, Irina Gurt, Naomi Kaisar-Iluz, Merav E. Shaul, Zvi G. Fridlender, Rivka Dresner-Pollak
International Journal of Molecular Sciences.2023; 24(13): 10526. CrossRef - Immune‐related adverse events after immune check point inhibitors: Understanding the intersection with autoimmunity
Namrata Singh, Anne M. Hocking, Jane H. Buckner
Immunological Reviews.2023; 318(1): 81. CrossRef - Endocrine Side Effects in Patients Treated with Immune Checkpoint Inhibitors: A Narrative Review
Nicia I. Profili, Roberto Castelli, Antonio Gidaro, Alessandro Merella, Roberto Manetti, Giuseppe Palmieri, Margherita Maioli, Alessandro P. Delitala
Journal of Clinical Medicine.2023; 12(15): 5161. CrossRef - Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
Endocrinology and Metabolism.2023; 38(6): 750. CrossRef - PD-1/PD-L1 Inhibitors in Patients With Preexisting Autoimmune Diseases
Ke Zhang, Xiangyi Kong, Yuan Li, Zhongzhao Wang, Lin Zhang, Lixue Xuan
Frontiers in Pharmacology.2022;[Epub] CrossRef - Association between the type of thyroid dysfunction induced by immune checkpoint inhibitors and prognosis in cancer patients
Han-sang Baek, Chaiho Jeong, Kabsoo Shin, Jaejun Lee, Heysun Suh, Dong-Jun Lim, Moo Il Kang, Jeonghoon Ha
BMC Endocrine Disorders.2022;[Epub] CrossRef - A Successful Case of Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab with Multisystem Immune-related Adverse Events
Hidemi Hayashi, Koji Sawada, Takumu Hasebe, Shunsuke Nakajima, Jun Sawada, Yuri Takiyama, Yumi Takiyama, Toshikatsu Okumura, Mikihiro Fujiya
Internal Medicine.2022; 61(23): 3497. CrossRef - Immune Related Adverse Events of the Thyroid – A Narrative Review
Christopher A. Muir, Venessa H. M. Tsang, Alexander M. Menzies, Roderick J. Clifton-Bligh
Frontiers in Endocrinology.2022;[Epub] CrossRef - Thyroid-related adverse events induced by immune checkpoint inhibitors
Alexandra Chera, Andreea Lucia Stancu, Octavian Bucur
Frontiers in Endocrinology.2022;[Epub] CrossRef - Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors
Adithya Chennamadhavuni, Laith Abushahin, Ning Jin, Carolyn J. Presley, Ashish Manne
Frontiers in Immunology.2022;[Epub] CrossRef - Case 5: A 41-Year-Old Woman With Palpitation
Jiwon Yang, Kabsoo Shin, Jeongmin Lee, Jeonghoon Ha, Dong-Jun Lim, Han-Sang Baek
Journal of Korean Medical Science.2022;[Epub] CrossRef - Immune Checkpoint Inhibitors and Endocrine Disorders: A Position Statement from the Korean Endocrine Society
Hyemi Kwon, Eun Roh, Chang Ho Ahn, Hee Kyung Kim, Cheol Ryong Ku, Kyong Yeun Jung, Ju Hee Lee, Eun Heui Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Jun Sung Moon, Jin Hwa Kim, Mi-kyung Kim
Endocrinology and Metabolism.2022; 37(6): 839. CrossRef - Management of Endocrine and Metabolic Toxicities of Immune-Checkpoint Inhibitors: From Clinical Studies to a Real-Life Scenario
Calogera Claudia Spagnolo, Giuseppe Giuffrida, Salvatore Cannavò, Tindara Franchina, Nicola Silvestris, Rosaria Maddalena Ruggeri, Mariacarmela Santarpia
Cancers.2022; 15(1): 246. CrossRef - Antineoplastics
Reactions Weekly.2021; 1874(1): 31. CrossRef
- Thyroid Dysfunction after Atezolizumab and Bevacizumab Is Associated with Favorable Outcomes in Hepatocellular Carcinoma


 KES
KES

 First
First Prev
Prev



