Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- The Road towards Triple Agonists: Glucagon-Like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide and Glucagon Receptor - An Update
- Agnieszka Jakubowska, Carel W. le Roux, Adie Viljoen
- Endocrinol Metab. 2024;39(1):12-22. Published online February 14, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1942
- 2,541 View
- 204 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
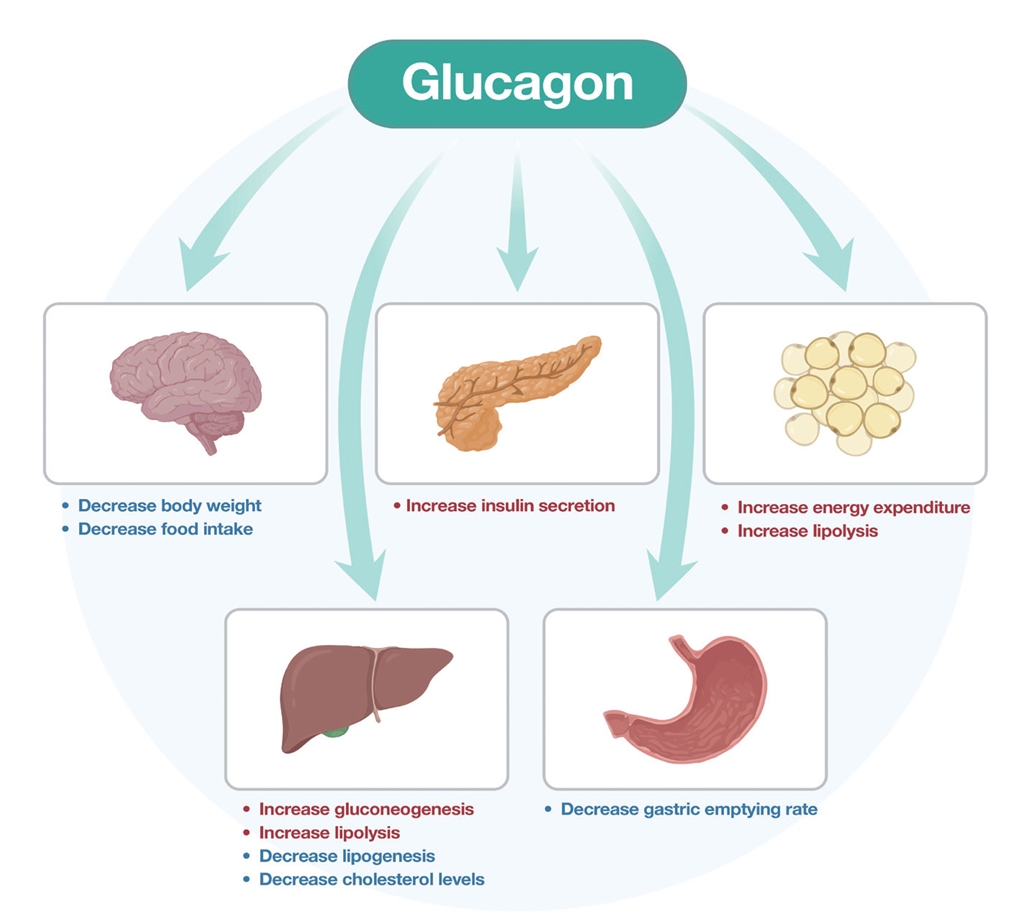
ePub - Obesity is the fifth leading risk factor for global deaths with numbers continuing to increase worldwide. In the last 20 years, the emergence of pharmacological treatments for obesity based on gastrointestinal hormones has transformed the therapeutic landscape. The successful development of glucagon-like peptide-1 (GLP-1) receptor agonists, followed by the synergistic combined effect of glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonists achieved remarkable weight loss and glycemic control in those with the diseases of obesity and type 2 diabetes. The multiple cardiometabolic benefits include improving glycemic control, lipid profiles, blood pressure, inflammation, and hepatic steatosis. The 2023 phase 2 double-blind, randomized controlled trial evaluating a GLP-1/GIP/glucagon receptor triagonist (retatrutide) in patients with the disease of obesity reported 24.2% weight loss at 48 weeks with 12 mg retatrutide. This review evaluates the current available evidence for GLP-1 receptor agonists, dual GLP-1/GIP receptor co-agonists with a focus on GLP-1/GIP/glucagon receptor triagonists and discusses the potential future benefits and research directions.
-
Citations
Citations to this article as recorded by- New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review
Jorge E. Jalil, Luigi Gabrielli, María Paz Ocaranza, Paul MacNab, Rodrigo Fernández, Bruno Grassi, Paulina Jofré, Hugo Verdejo, Monica Acevedo, Samuel Cordova, Luis Sanhueza, Douglas Greig
International Journal of Molecular Sciences.2024; 25(8): 4407. CrossRef
- New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review

- Diabetes, Obesity and Metabolism
- Incretin and Pancreatic β-Cell Function in Patients with Type 2 Diabetes
- Chang Ho Ahn, Tae Jung Oh, Se Hee Min, Young Min Cho
- Endocrinol Metab. 2023;38(1):1-9. Published online February 13, 2023
- DOI: https://doi.org/10.3803/EnM.2023.103
- 3,318 View
- 359 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
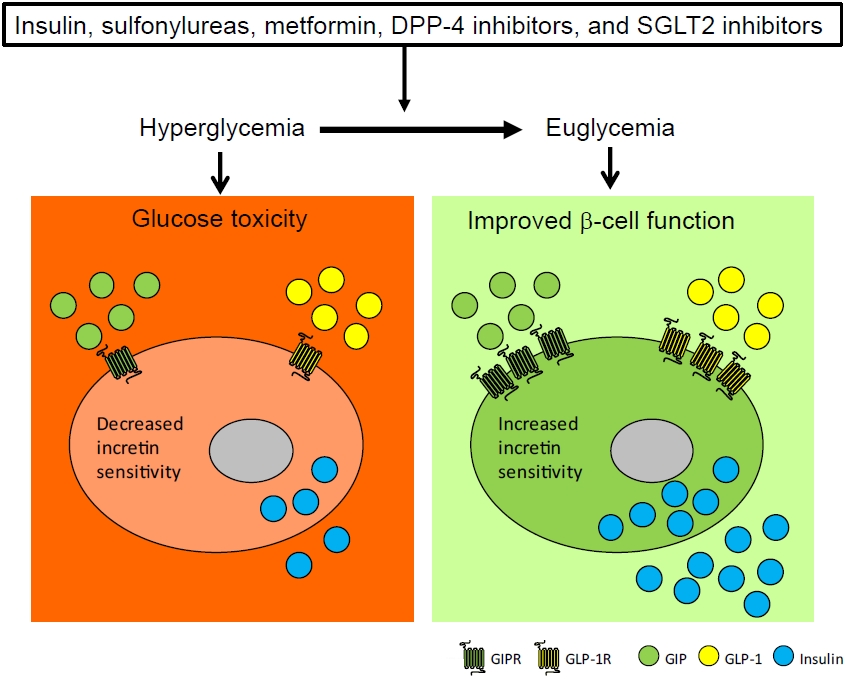
ePub - To maintain normal glucose homeostasis after a meal, it is essential to secrete an adequate amount of insulin from pancreatic β-cells. However, if pancreatic β-cells solely depended on the blood glucose level for insulin secretion, a surge in blood glucose levels would be inevitable after the ingestion of a large amount of carbohydrates. To avoid a deluge of glucose in the bloodstream after a large carbohydrate- rich meal, enteroendocrine cells detect the amount of nutrient absorption from the gut lumen and secrete incretin hormones at scale. Since insulin secretion in response to incretin hormones occurs only in a hyperglycemic milieu, pancreatic β-cells can secrete a “Goldilocks” amount of insulin (i.e., not too much and not too little) to keep the blood glucose level in the normal range. In this regard, pancreatic β-cell sensitivity to glucose and incretin hormones is crucial for maintaining normal glucose homeostasis. In this Namgok lecture 2022, we review the effects of current anti-diabetic medications on pancreatic β-cell sensitivity to glucose and incretin hormones.
-
Citations
Citations to this article as recorded by- Initial Combination Therapy in Type 2 Diabetes
Ji Yoon Kim, Nam Hoon Kim
Endocrinology and Metabolism.2024; 39(1): 23. CrossRef
- Initial Combination Therapy in Type 2 Diabetes

- Diabetes, Obesity and Metabolism
- Glucagon-Like Peptide 1 Therapy: From Discovery to Type 2 Diabetes and Beyond
- Adie Viljoen, Stephen C. Bain
- Endocrinol Metab. 2023;38(1):25-33. Published online February 6, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1642
- 2,762 View
- 305 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The therapeutic benefits of the incretin hormone, glucagon-like peptide 1 (GLP1), for people with type 2 diabetes and/or obesity, are now firmly established. The evidence-base arising from head-to-head comparative effectiveness studies in people with type 2 diabetes, as well as the recommendations by professional guidelines suggest that GLP1 receptor agonists should replace more traditional treatment options such as sulfonylureas and dipeptidyl-peptidase 4 (DPP4) inhibitors. Furthermore, their benefits in reducing cardiovascular events in people with type 2 diabetes beyond improvements in glycaemic control has led to numerous clinical trials seeking to translate this benefit beyond type 2 diabetes. Following early trial results their therapeutic benefit is currently being tested in other conditions including fatty liver disease, kidney disease, and Alzheimer’s disease.
-
Citations
Citations to this article as recorded by- The Road towards Triple Agonists: Glucagon-Like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide and Glucagon Receptor - An Update
Agnieszka Jakubowska, Carel W. le Roux, Adie Viljoen
Endocrinology and Metabolism.2024; 39(1): 12. CrossRef - Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action
John R. Ussher, Daniel J. Drucker
Nature Reviews Cardiology.2023; 20(7): 463. CrossRef - A new class of glucose-lowering therapy for type 2 diabetes: the latest development in the incretin arena
Stephen C Bain, Thinzar Min
The Lancet.2023; 402(10401): 504. CrossRef - Flattening the biological age curve by improving metabolic health: to taurine or not to taurine, that’ s the question
Kwok M. Ho, Anna Lee, William Wu, Matthew T.V. Chan, Lowell Ling, Jeffrey Lipman, Jason Roberts, Edward Litton, Gavin M. Joynt, Martin Wong
Journal of Geriatric Cardiology.2023; 20(11): 813. CrossRef
- The Road towards Triple Agonists: Glucagon-Like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide and Glucagon Receptor - An Update

- Diabetes, Obesity and Metabolism
- Efficacy and Safety of the New Appetite Suppressant, Liraglutide: A Meta-Analysis of Randomized Controlled Trials
- Shinje Moon, Jibeom Lee, Hye Soo Chung, Yoon Jung Kim, Jae Myung Yu, Sung Hoon Yu, Chang-Myung Oh
- Endocrinol Metab. 2021;36(3):647-660. Published online June 18, 2021
- DOI: https://doi.org/10.3803/EnM.2020.934

- 6,136 View
- 301 Download
- 13 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Obesity is a chronic disease associated with metabolic diseases such as diabetes and cardiovascular disease. Since the U.S. Food and Drug Administration approved liraglutide as an anti-obesity drug for nondiabetic patients in 2014, it has been widely used for weight control in overweight and obese people. This study aimed to systematically analyze the effects of liraglutide on body weight and other cardiometabolic parameters.
Methods
We investigated articles from PubMed, EMBASE, and the Cochrane Library to search randomized clinical trials that examined body weight changes with liraglutide treatment.
Results
We included 31 studies with 8,060 participants for this meta-analysis. The mean difference (MD) between the liraglutide group and the placebo group was −4.19 kg (95% confidence interval [CI], −4.84 to −3.55), with a −4.16% change from the baseline (95% CI, −4.90 to −3.43). Liraglutide treatment correlated with a significantly reduced body mass index (MD: −1.55; 95% CI, −1.76 to −1.34) and waist circumference (MD: −3.11 cm; 95% CI, −3.59 to −2.62) and significantly decreased blood pressure (systolic blood pressure, MD: −2.85 mm Hg; 95% CI, −3.36 to −2.35; diastolic blood pressure, MD: −0.66 mm Hg; 95% CI, −1.02 to −0.30), glycated hemoglobin (MD: −0.40%; 95% CI, −0.49 to −0.31), and low-density lipoprotein cholesterol (MD: –2.91 mg/dL; 95% CI, −5.28 to −0.53; MD: −0.87% change from baseline; 95% CI, −1.17 to −0.56).
Conclusion
Liraglutide is effective for weight control and can be a promising drug for cardiovascular protection in overweight and obese people. -
Citations
Citations to this article as recorded by- Pharmacotherapy for obesity: moving towards efficacy improvement
Walmir Coutinho, Bruno Halpern
Diabetology & Metabolic Syndrome.2024;[Epub] CrossRef - Physiopathology and Treatment of Obesity and Overweight: A Proposal for a New Anorectic
Bruno Silvestrini, Mauro Silvestrini, Mayank Choubey
Journal of Obesity.2024; 2024: 1. CrossRef - Side effect profile of pharmacologic therapies for liver fibrosis in nonalcoholic fatty liver disease: a systematic review and network meta-analysis
Yilin Li, Rong Lei, Honglin Lei, Qin Xiong, Fengjiao Xie, Chengjiao Yao, Peimin Feng
European Journal of Gastroenterology & Hepatology.2023; 35(1): 1. CrossRef - Recommendations for the prevention and management of obesity in the Iraqi population
Hussein Ali Nwayyir, Esraa Majid Mutasher, Osama Mohammed Alabid, Muthana Abdulrazzaq Jabbar, Wefak Hasan Abdulraheem Al-Kawaz, Haider Ayad Alidrisi, Majid Alabbood, Muhammed Chabek, Munib AlZubaidi, Lujain Anwar Al-khazrajy, Ibtihal Shukri Abd Alhaleem,
Postgraduate Medicine.2023; 135(5): 425. CrossRef - A Comprehensive Review on Weight Loss Associated with Anti-Diabetic Medications
Fatma Haddad, Ghadeer Dokmak, Maryam Bader, Rafik Karaman
Life.2023; 13(4): 1012. CrossRef - Liraglutide, a glucagon-like peptide-1 analog, in individuals with obesity in clinical practice
Juyoung Shin, Raeun Kim, Hun-Sung Kim
Cardiovascular Prevention and Pharmacotherapy.2023; 5(2): 49. CrossRef - The effects of subcutaneous Tirzepatide on obesity and overweight: a systematic review and meta‐regression analysis of randomized controlled trials
Pejman Rohani, Nasser Malekpour Alamdari, Seyedeh Elaheh Bagheri, Azita Hekmatdoost, Mohammad Hassan Sohouli
Frontiers in Endocrinology.2023;[Epub] CrossRef - Efficacy and safety of liraglutide for weight management in children and adolescents: a systematic review and meta-analysis of randomized controlled trials
Hao Gou, Yiman Zhai, Junjun Guo
European Journal of Pediatrics.2023; 182(11): 5095. CrossRef - Efficacy and safety of once-weekly semaglutide in adults with overweight or obesity: a meta-analysis
Ping Zhong, Hai Zeng, Miaochun Huang, Wenbin Fu, Zhixia Chen
Endocrine.2022; 75(3): 718. CrossRef - Pharmacological profile of once-weekly injectable semaglutide for chronic weight management
David C. W. Lau, Rachel L Batterham, Carel W. le Roux
Expert Review of Clinical Pharmacology.2022; 15(3): 251. CrossRef - Pharmacological Management of Obesity: A Century of Expert Opinions in Cecil Textbook of Medicine
Peter Manu, Cristina-Mihaela Lăcătuşu, Liliana M. Rogozea, Simona Cernea
American Journal of Therapeutics.2022; 29(4): e410. CrossRef - GLP-1 agonists: superior for mind and body in antipsychotic-treated patients?
Katerina Horska, Jana Ruda-Kucerova, Silje Skrede
Trends in Endocrinology & Metabolism.2022; 33(9): 628. CrossRef - Targeting skeletal muscle mitochondrial health in obesity
Chantal A. Pileggi, Breana G. Hooks, Ruth McPherson, Robert R.M. Dent, Mary-Ellen Harper
Clinical Science.2022; 136(14): 1081. CrossRef - A Study on Weight Loss Cause as per the Side Effect of Liraglutide
Jin Yu, Jeongmin Lee, Seung-Hwan Lee, Jae-Hyung Cho, Hun-Sung Kim, Heng Zhou
Cardiovascular Therapeutics.2022; 2022: 1. CrossRef
- Pharmacotherapy for obesity: moving towards efficacy improvement

- Diabetes
- Peptidyl and Non-Peptidyl Oral Glucagon-Like Peptide-1 Receptor Agonists
- Hun Jee Choe, Young Min Cho
- Endocrinol Metab. 2021;36(1):22-29. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2021.102
- 27,839 View
- 654 Download
- 11 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
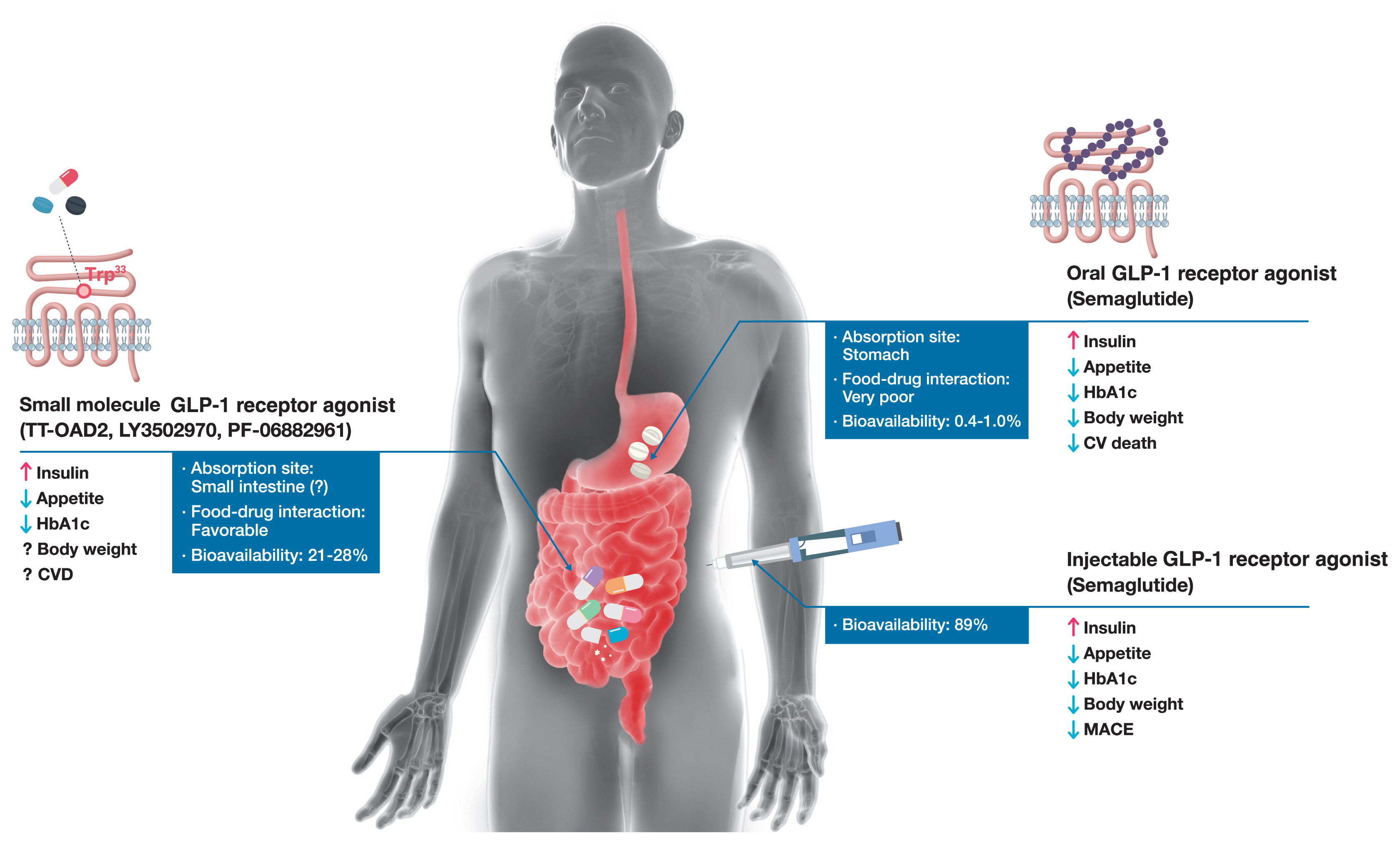
ePub - Glucagon-like peptide-1 (GLP-1) receptor agonists are efficacious glucose-lowering medications with salient benefits for body weight and cardiovascular events. This class of medications is now recommended as the top priority for patients with established cardiovascular disease or indicators of high risk. Until the advent of oral semaglutide, however, GLP-1 receptor agonists were available only in the form of subcutaneous injections. Aversion to needles, discomfort with self-injection, or skin problems at the injection site are commonly voiced problems in people with diabetes, and thus, attempts for non-invasive delivery strategies have continued. Herein, we review the evolution of GLP-1 therapy from its discovery and the development of currently approved drugs to the unprecedented endeavor to administer GLP-1 receptor agonists via the oral route. We focus on the pharmacokinetic and pharmacodynamic properties of the recently approved oral GLP-1 receptor agonist, oral semaglutide. Small molecule oral GLP-1 receptor agonists are currently in development, and we introduce how these chemicals have addressed the challenge posed by interactions with the large extracellular ligand binding domain of the GLP-1 receptor. We specifically discuss the structure and pharmacological properties of TT-OAD2, LY3502970, and PF-06882961, and envision an era where more patients could benefit from oral GLP-1 receptor agonist therapy.
-
Citations
Citations to this article as recorded by- Sulfobetaine modification of poly (D, L-lactide-co-glycolic acid) nanoparticles enhances mucus permeability and improves bioavailability of orally delivered liraglutide
Zhenyu Zhao, Ruihuan Ding, Yumei Wang, Ranran Yuan, Houqian Zhang, Tianyang Li, Wei Zheng, Entao Chen, Aiping Wang, Yanan Shi
Journal of Drug Delivery Science and Technology.2024; 93: 105437. CrossRef - Physiology and pharmacology of glucagon-like peptide-1 receptor
D. V. Kurkin, D. A. Bakulin, E. I. Morkovin, V. I. Petrov, A. V. Strygin, K. N. Koryanova, Yu. V. Gorbunova, Yu. A. Kolosov, O. V. Ivanova, E. V. Pavlova, M. A. Dzhavakhyan, A. V. Zaborovsky, V. B. Saparova, I. E. Makarenko, R. I. Drai, A. N. Chumachenko
Pharmacy & Pharmacology.2024; 11(4): 347. CrossRef - G protein-coupled receptors driven intestinal glucagon-like peptide-1 reprogramming for obesity: Hope or hype?
Mohan Patil, Ilaria Casari, Leon N. Warne, Marco Falasca
Biomedicine & Pharmacotherapy.2024; 172: 116245. CrossRef - Glucagon-like peptide-1 analogs: Miracle drugs are blooming?
Binbin Gong, Zhihong Yao, Chenxu Zhou, Wenxi Wang, Lidan Sun, Jing Han
European Journal of Medicinal Chemistry.2024; 269: 116342. CrossRef - Opportunities and challenges of incretin-based hypoglycemic agents treating type 2 diabetes mellitus from the perspective of physiological disposition
Yaochen Xie, Qian Zhou, Qiaojun He, Xiaoyi Wang, Jincheng Wang
Acta Pharmaceutica Sinica B.2023; 13(6): 2383. CrossRef - Advances in GLP-1 receptor agonists for the treatment of type 2 diabetes
Shurui Hong, J. Xiao, Y. He
BIO Web of Conferences.2023; 61: 01006. CrossRef - Safety and efficacy of the new, oral, small-molecule, GLP-1 receptor agonists orforglipron and danuglipron for the treatment of type 2 diabetes and obesity: systematic review and meta-analysis of randomized controlled trials
Paschalis Karakasis, Dimitrios Patoulias, Konstantinos Pamporis, Panagiotis Stachteas, Konstantinos I. Bougioukas, Aleksandra Klisic, Nikolaos Fragakis, Manfredi Rizzo
Metabolism.2023; 149: 155710. CrossRef - A review of glucoregulatory hormones potentially applicable to the treatment of Alzheimer’s disease: mechanism and brain delivery
Reeju Amatya, Kyoung Ah Min, Meong Cheol Shin
Journal of Pharmaceutical Investigation.2022; 52(2): 195. CrossRef - Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022
Harold E. Bays, Angela Fitch, Sandra Christensen, Karli Burridge, Justin Tondt
Obesity Pillars.2022; 2: 100018. CrossRef - Structural basis of peptidomimetic agonism revealed by small-molecule GLP-1R agonists Boc5 and WB4-24
Zhaotong Cong, Qingtong Zhou, Yang Li, Li-Nan Chen, Zi-Chen Zhang, Anyi Liang, Qing Liu, Xiaoyan Wu, Antao Dai, Tian Xia, Wei Wu, Yan Zhang, Dehua Yang, Ming-Wei Wang
Proceedings of the National Academy of Sciences.2022;[Epub] CrossRef - Improved Split TEV GPCR β-arrestin-2 Recruitment Assays via Systematic Analysis of Signal Peptide and β-arrestin Binding Motif Variants
Yuxin Wu, Isabelle von Hauff, Niels Jensen, Moritz Rossner, Michael Wehr
Biosensors.2022; 13(1): 48. CrossRef - GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects
Xin Zhao, Minghe Wang, Zhitong Wen, Zhihong Lu, Lijuan Cui, Chao Fu, Huan Xue, Yunfeng Liu, Yi Zhang
Frontiers in Endocrinology.2021;[Epub] CrossRef - Recent developments in GLP‐1RA therapy: A review of the latest evidence of efficacy and safety and differences within the class
Evie K. Bain, Stephen C. Bain
Diabetes, Obesity and Metabolism.2021; 23(S3): 30. CrossRef
- Sulfobetaine modification of poly (D, L-lactide-co-glycolic acid) nanoparticles enhances mucus permeability and improves bioavailability of orally delivered liraglutide

- Endocrine Research
- Effects of Glucagon-Like Peptide-1 Analogue and Fibroblast Growth Factor 21 Combination on the Atherosclerosis-Related Process in a Type 2 Diabetes Mouse Model
- Jin Hee Kim, Gha Young Lee, Hyo Jin Maeng, Hoyoun Kim, Jae Hyun Bae, Kyoung Min Kim, Soo Lim
- Endocrinol Metab. 2021;36(1):157-170. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2020.781
- 6,832 View
- 175 Download
- 10 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Glucagon-like peptide-1 (GLP-1) analogues regulate glucose homeostasis and have anti-inflammatory properties, but cause gastrointestinal side effects. The fibroblast growth factor 21 (FGF21) is a hormonal regulator of lipid and glucose metabolism that has poor pharmacokinetic properties, including a short half-life. To overcome these limitations, we investigated the effect of a low-dose combination of a GLP-1 analogue and FGF21 on atherosclerosis-related molecular pathways.
Methods
C57BL/6J mice were fed a high-fat diet for 30 weeks followed by an atherogenic diet for 10 weeks and were divided into four groups: control (saline), liraglutide (0.3 mg/kg/day), FGF21 (5 mg/kg/day), and low-dose combination treatment with liraglutide (0.1 mg/kg/day) and FGF21 (2.5 mg/kg/day) (n=6/group) for 6 weeks. The effects of each treatment on various atherogenesisrelated pathways were assessed.
Results
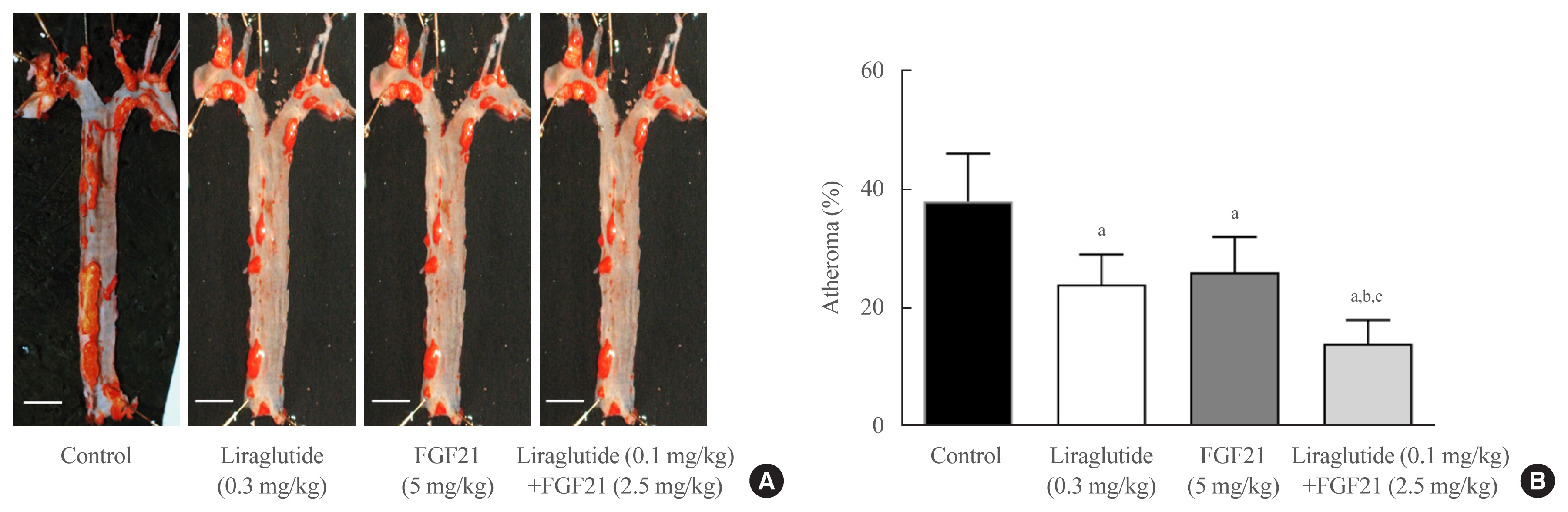
Liraglutide, FGF21, and their low-dose combination significantly reduced atheromatous plaque in aorta, decreased weight, glucose, and leptin levels, and increased adiponectin levels. The combination treatment upregulated the hepatic uncoupling protein-1 (UCP1) and Akt1 mRNAs compared with controls. Matric mentalloproteinase-9 (MMP-9), monocyte chemoattractant protein-1 (MCP-1), and intercellular adhesion molecule-1 (ICAM-1) were downregulated and phosphorylated Akt (p-Akt) and phosphorylated extracellular signal-regulated kinase (p-ERK) were upregulated in liver of the liraglutide-alone and combination-treatment groups. The combination therapy also significantly decreased the proliferation of vascular smooth muscle cells. Caspase-3 was increased, whereas MMP-9, ICAM-1, p-Akt, and p-ERK1/2 were downregulated in the liraglutide-alone and combination-treatment groups.
Conclusion
Administration of a low-dose GLP-1 analogue and FGF21 combination exerts beneficial effects on critical pathways related to atherosclerosis, suggesting the synergism of the two compounds. -
Citations
Citations to this article as recorded by- Current status and future perspectives of FGF21 analogues in clinical trials
Zara Siu Wa Chui, Qing Shen, Aimin Xu
Trends in Endocrinology & Metabolism.2024;[Epub] CrossRef - Design and pharmaceutical evaluation of bifunctional fusion protein of FGF21 and GLP-1 in the treatment of nonalcoholic steatohepatitis
Xianlong Ye, Yingli Chen, Jianying Qi, Shenglong Zhu, Yuanyuan Wu, Jingjing Xiong, Fei Hu, Zhimou Guo, Xinmiao Liang
European Journal of Pharmacology.2023; 952: 175811. CrossRef - Use of FGF21 analogs for the treatment of metabolic disorders: a systematic review and meta-analysis
Maria Paula Carbonetti, Fernanda Almeida-Oliveira, David Majerowicz
Archives of Endocrinology and Metabolism.2023;[Epub] CrossRef - Exploring the potential mechanism of Simiao Yongan decoction in the treatment of diabetic peripheral vascular disease based on network pharmacology and molecular docking technology
Fang Cao, Yongkang Zhang, Yuan Zong, Xia Feng, Junlin Deng, Yuzhen Wang, Yemin Cao
Medicine.2023; 102(52): e36762. CrossRef - The Healing Capability of Clove Flower Extract (CFE) in Streptozotocin-Induced (STZ-Induced) Diabetic Rat Wounds Infected with Multidrug Resistant Bacteria
Rewaa Ali, Tarek Khamis, Gamal Enan, Gamal El-Didamony, Basel Sitohy, Gamal Abdel-Fattah
Molecules.2022; 27(7): 2270. CrossRef - Nonalcoholic Steatohepatitis (NASH) and Atherosclerosis: Explaining Their Pathophysiology, Association and the Role of Incretin-Based Drugs
Eleftheria Galatou, Elena Mourelatou, Sophia Hatziantoniou, Ioannis S. Vizirianakis
Antioxidants.2022; 11(6): 1060. CrossRef - Unlocking the Therapeutic Potential of Glucagon-Like Peptide-1 Analogue and Fibroblast Growth Factor 21 Combination for the Pathogenesis of Atherosclerosis in Type 2 Diabetes
Jang Won Son
Endocrinology and Metabolism.2021; 36(1): 57. CrossRef - Effects of fasting on skeletal muscles and body fat of adult and old C57BL/6J mice
Mindaugas Kvedaras, Petras Minderis, Leonardo Cesanelli, Agne Cekanauskaite, Aivaras Ratkevicius
Experimental Gerontology.2021; 152: 111474. CrossRef - The Role of Fibroblast Growth Factor 21 in Diabetic Cardiovascular Complications and Related Epigenetic Mechanisms
Mengjie Xiao, Yufeng Tang, Shudong Wang, Jie Wang, Jie Wang, Yuanfang Guo, Jingjing Zhang, Junlian Gu
Frontiers in Endocrinology.2021;[Epub] CrossRef - Liraglutide Decreases Liver Fat Content and Serum Fibroblast Growth Factor 21 Levels in Newly Diagnosed Overweight Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease
Xinyue Li, Xiaojuan Wu, Yumei Jia, Jing Fu, Lin Zhang, Tao Jiang, Jia Liu, Guang Wang, Claudia Cardoso
Journal of Diabetes Research.2021; 2021: 1. CrossRef - Differential importance of endothelial and hematopoietic cell GLP-1Rs for cardiometabolic versus hepatic actions of semaglutide
Brent A. McLean, Chi Kin Wong, Kiran Deep Kaur, Randy J. Seeley, Daniel J. Drucker
JCI Insight.2021;[Epub] CrossRef
- Current status and future perspectives of FGF21 analogues in clinical trials

- Effects of Incretin-Based Therapies on Diabetic Microvascular Complications
- Yu Mi Kang, Chang Hee Jung
- Endocrinol Metab. 2017;32(3):316-325. Published online September 18, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.3.316
- 4,556 View
- 55 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The morbidity and mortality associated with diabetic complications impose a huge socioeconomic burden worldwide. Therefore, the ultimate goal of managing diabetes mellitus (DM) is to lower the risk of macrovascular complications and highly morbid microvascular complications such as diabetic nephropathy (DN) and diabetic retinopathy (DR). Potential benefits of incretin-based therapies such as glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and dipeptidyl peptidase-4 (DPP-4) inhibitors on the diabetic macrovascular complications have been recently suggested, owing to their pleiotropic effects on multiple organ systems. However, studies primarily investigating the role of these therapies in diabetic microvascular complications are rare. Nevertheless, preclinical and limited clinical data suggest the potential protective effect of incretin-based agents against DN and DR via their anti-inflammatory, antioxidative, and antiapoptotic properties. Evidence also suggests that these incretin-dependent and independent beneficial effects are not necessarily associated with the glucose-lowering properties of GLP-1 RAs and DPP-4 inhibitors. Hence, in this review, we revisit the preclinical and clinical evidence of incretin-based therapy for DR and DN, the two most common, morbid complications in individuals with DM. In addition, the review discusses a few recent studies raising concerns of aggravating DR with the use of incretin-based therapies.
-
Citations
Citations to this article as recorded by- Efficacy and Safety of the Utilization of Dipeptidyl Peptidase IV Inhibitors in Diabetic Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials
Moeber Mahzari, Muhannad Alqirnas, Moustafa Alhamadh, Faisal Alrasheed, Abdulrahman Alhabeeb, Wedad Al Madani, Hussain Aldera
Diabetes, Metabolic Syndrome and Obesity.2024; Volume 17: 1425. CrossRef - Anti-Inflammatory Effects of GLP-1R Activation in the Retina
Alessandra Puddu, Davide Maggi
International Journal of Molecular Sciences.2022; 23(20): 12428. CrossRef - Diabetes and Its Complications: Therapies Available, Anticipated and Aspired
Anu Grover, Komal Sharma, Suresh Gautam, Srishti Gautam, Monica Gulati, Sachin Kumar Singh
Current Diabetes Reviews.2021; 17(4): 397. CrossRef - SGLT2 Inhibitors, GLP-1 Agonists, and DPP-4 Inhibitors in Diabetes and Microvascular Complications: A Review
Christopher El Mouhayyar, Ruba Riachy, Abir Bou Khalil, Asaad Eid, Sami Azar
International Journal of Endocrinology.2020; 2020: 1. CrossRef - Novel therapeutic agents for the treatment of diabetic kidney disease
Rachel E. Hartman, P.S.S. Rao, Mariann D. Churchwell, Susan J. Lewis
Expert Opinion on Investigational Drugs.2020; 29(11): 1277. CrossRef - Nationwide Trends in Pancreatitis and Pancreatic Cancer Risk Among Patients With Newly Diagnosed Type 2 Diabetes Receiving Dipeptidyl Peptidase 4 Inhibitors
Minyoung Lee, Jiyu Sun, Minkyung Han, Yongin Cho, Ji-Yeon Lee, Chung Mo Nam, Eun Seok Kang
Diabetes Care.2019; 42(11): 2057. CrossRef - Effects of Dipeptidyl Peptidase-4 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis
Jae Hyun Bae, Sunhee Kim, Eun-Gee Park, Sin Gon Kim, Seokyung Hahn, Nam Hoon Kim
Endocrinology and Metabolism.2019; 34(1): 80. CrossRef - Serum adipocytokines are associated with microalbuminuria in patients with type 1 diabetes and incipient chronic complications
Tomislav Bulum, Marijana Vučić Lovrenčić, Martina Tomić, Sandra Vučković-Rebrina, Vinko Roso, Branko Kolarić, Vladimir Vuksan, Lea Duvnjak
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(1): 496. CrossRef - Protective Effects of Incretin Against Age-Related Diseases
Di Zhang, Mingzhu Ma, Yueze Liu
Current Drug Delivery.2019; 16(9): 793. CrossRef - The role of dipeptidylpeptidase-4 inhibitors in management of cardiovascular disease in diabetes; focus on linagliptin
Annayya R. Aroor, Camila Manrique-Acevedo, Vincent G. DeMarco
Cardiovascular Diabetology.2018;[Epub] CrossRef
- Efficacy and Safety of the Utilization of Dipeptidyl Peptidase IV Inhibitors in Diabetic Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials

- Clinical Study
- Correlation of Glypican-4 Level with Basal Active Glucagon-Like Peptide 1 Level in Patients with Type 2 Diabetes Mellitus
- Sang Ah Lee, Gwanpyo Koh, Suk Ju Cho, So-Yeon Yoo, Sang Ouk Chin
- Endocrinol Metab. 2016;31(3):439-445. Published online September 26, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.3.439
- 4,030 View
- 41 Download
- 8 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Previous studies have reported that glypican-4 (GPC4) regulates insulin signaling by interacting with insulin receptor and through adipocyte differentiation. However, GPC4 has not been studied with regard to its effects on clinical factors in patients with type 2 diabetes mellitus (T2DM). We aimed to identify factors associated with GPC4 level in T2DM.
Methods Between January 2010 and December 2013, we selected 152 subjects with T2DM and collected serum and plasma into tubes pretreated with aprotinin and dipeptidyl peptidase-4 inhibitor to preserve active gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). GPC4, active GLP-1, active GIP, and other factors were measured in these plasma samples. We performed a linear regression analysis to identify factors associated with GPC4 level.
Results The subjects had a mean age of 58.1 years, were mildly obese (mean body mass index [BMI], 26.1 kg/m2), had T2DM of long-duration (mean, 101.3 months), glycated hemoglobin 7.5%, low insulin secretion, and low insulin resistance (mean homeostatic model assessment of insulin resistance [HOMA-IR], 1.2). Their mean GPC4 was 2.0±0.2 ng/mL. In multivariate analysis, GPC4 was independently associated with age (β=0.224,
P =0.009), and levels of active GLP-1 (β=0.171,P =0.049) and aspartate aminotransferase (AST; β=–0.176,P =0.043) after being adjusted for other clinical factors.Conclusion GPC4 was independently associated with age, active GLP-1, and AST in T2DM patients, but was not associated with HOMA-IR and BMI, which are well known factors related to GPC4. Further study is needed to identify the mechanisms of the association between GPC4 and basal active GLP-1 levels.
-
Citations
Citations to this article as recorded by- How Reliable are Commercially Available Glypican4 ELISA
Kits?
Joseph P. Buhl, Antje Garten, Jürgen Kratzsch, Wieland Kiess, Melanie Penke
Experimental and Clinical Endocrinology & Diabetes.2022; 130(02): 110. CrossRef - Serum glypican-4 is associated with the 10-year clinical outcome of patients with peripheral artery disease
Axel Muendlein, Christine Heinzle, Andreas Leiherer, Kathrin Geiger, Eva Maria Brandtner, Stella Gaenger, Peter Fraunberger, Christoph H. Saely, Heinz Drexel
International Journal of Cardiology.2022; 369: 54. CrossRef - Berberine activates the β-catenin/TCF4 signaling pathway by down-regulating miR-106b to promote GLP-1 production by intestinal L cells
Jiao Wang, Li-Rui Wei, Yan-Ling Liu, Cheng-Zhi Ding, Feng Guo, Jiao Wang, Qian Qin, Feng-Jiao Huang, Ying Xin, Sheng-Nan Ma, Qiu-Ran Zhai, Shou-Jun Wang, Gui-Jun Qin
European Journal of Pharmacology.2021; 911: 174482. CrossRef - Increased Glypican-4 Levels Are Associated with Obesity in Adolescents
Huseyin Dag, Nevin Cetin Dag, Okan Dikker
Iranian Journal of Pediatrics.2019;[Epub] CrossRef - Serum glypican 4 level in obese children and its relation to degree of obesity
Chutima Leelalertlauw, Manassawee Korwutthikulrangsri, Pat Mahachoklertwattana, Suwannee Chanprasertyothin, Patcharin Khlairit, Sarunyu Pongratanakul, Preamrudee Poomthavorn
Clinical Endocrinology.2017; 87(6): 689. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - Efficacy and Safety of Single‐ or Double‐Drug Antidiabetic Regimens in the Treatment of Type 2 Diabetes Mellitus: A Network Meta‐Analysis
Xi‐Ling Yang, Mi‐Ma Duo‐Ji, Zi‐Wen Long
Journal of Cellular Biochemistry.2017; 118(12): 4536. CrossRef
- How Reliable are Commercially Available Glypican4 ELISA
Kits?

- Obesity and Metabolism
- Cardiovascular Effects of Glucagon-Like Peptide-1 Receptor Agonists
- Yu Mi Kang, Chang Hee Jung
- Endocrinol Metab. 2016;31(2):258-274. Published online April 25, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.258
- 6,356 View
- 92 Download
- 31 Web of Science
- 33 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Glucagon-like peptide-1 (GLP-1) is a member of the proglucagon incretin family, and GLP-1 receptor agonists (RAs) have been introduced as a new class of antidiabetic medications in the past decade. The benefits of GLP-1 RAs are derived from their pleiotropic effects, which include glucose-dependent insulin secretion, suppressed glucagon secretion, and reduced appetite. Moreover, GLP-1 RAs also exert beneficial roles on multiple organ systems in which the GLP-1 receptors exist, including the cardiovascular system. Cardiovascular effects of GLP-1 RAs have been of great interest since the burden from cardiovascular diseases (CVD) has been unbearably increasing in a diabetic population worldwide, despite strict glycemic control and advanced therapeutic techniques to treat CVD. Preclinical studies have already demonstrated the beneficial effects of GLP-1 on myocardium and vascular endothelium, and many clinical studies evaluating changes in surrogate markers of CVD have suggested potential benefits from the use of GLP-1 RAs. Data from numerous clinical trials primarily evaluating the antihyperglycemic effects of multiple GLP-1 RAs have also revealed that changes in most CVD risk markers reported as secondary outcomes have been in favor of GLP-1 RAs treatment. However, to date, there is only one randomized clinical trial of GLP-1 RAs (the ELIXA study) evaluating major cardiovascular events as their primary outcomes, and in this study, a neutral cardiovascular effect of lixisenatide was observed in high-risk diabetic subjects. Therefore, the results of ongoing CVD outcome trials with the use of GLP-1 RAs should be awaited to elucidate the translation of benefits previously seen in CVD risk marker studies into large clinical trials with primary cardiovascular outcomes.
-
Citations
Citations to this article as recorded by- Cardioprotective Actions of a Glucagon‐like Peptide‐1 Receptor Agonist on Hearts Donated After Circulatory Death
Sachiko Kadowaki, M. Ahsan Siraj, Weiden Chen, Jian Wang, Marlee Parker, Anita Nagy, Chun‐Po Steve Fan, Kyle Runeckles, Jing Li, Junko Kobayashi, Christoph Haller, Mansoor Husain, Osami Honjo
Journal of the American Heart Association.2023;[Epub] CrossRef - The role of dipeptidyl peptidase-IV in abdominal aortic aneurysm pathogenesis: A systematic review
Elisha Ngetich, Pierfrancesco Lapolla, Anirudh Chandrashekar, Ashok Handa, Regent Lee
Vascular Medicine.2022; 27(1): 77. CrossRef - Glucagon-like Peptide-1 Receptor Agonists in the Management of Type 2 Diabetes Mellitus and Obesity: The Impact of Pharmacological Properties and Genetic Factors
Jasna Klen, Vita Dolžan
International Journal of Molecular Sciences.2022; 23(7): 3451. CrossRef - Glucagon-like peptide-1 (GLP-1) receptor agonists and cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of double-blind, randomized, placebo-controlled clinical trials
Jing Qin, Li Song
BMC Endocrine Disorders.2022;[Epub] CrossRef - Role of G-protein coupled receptor (GPCRs)/(GPR-120) as an agonists in diabetic wound healing
Jagat Pal Yadav, Dinesh Kumar Patel, Prateek Pathak, Maria Grishina
Obesity Medicine.2022; 36: 100466. CrossRef - Protection against stroke with glucagon-like peptide-1 receptor agonists: a comprehensive review of potential mechanisms
Bruno Vergès, Victor Aboyans, Denis Angoulvant, Pierre Boutouyrie, Bertrand Cariou, Fabien Hyafil, Kamel Mohammedi, Pierre Amarenco
Cardiovascular Diabetology.2022;[Epub] CrossRef - Changing Fields-Diabetes Medications Invading the Cardiovascular Space
Lauren D. Breite, Mackenzie Steck, Brandon Tate Cutshall, Samarth P. Shah, Brandon E. Cave
Current Problems in Cardiology.2021; 46(3): 100736. CrossRef - PEGDA/HA mineralized hydrogel loaded with Exendin4 promotes bone regeneration in rat models with bone defects by inducing osteogenesis
Wei Liu, Xiaowei Jing, Zhiwen Xu, Chong Teng
Journal of Biomaterials Applications.2021; 35(10): 1337. CrossRef - Metabolite G-Protein Coupled Receptors in Cardio-Metabolic Diseases
Derek Strassheim, Timothy Sullivan, David C. Irwin, Evgenia Gerasimovskaya, Tim Lahm, Dwight J. Klemm, Edward C. Dempsey, Kurt R. Stenmark, Vijaya Karoor
Cells.2021; 10(12): 3347. CrossRef - PPG neurons in the nucleus of the solitary tract modulate heart rate but do not mediate GLP-1 receptor agonist-induced tachycardia in mice
Marie K. Holt, Daniel R. Cook, Daniel I. Brierley, James E. Richards, Frank Reimann, Alexander V. Gourine, Nephtali Marina, Stefan Trapp
Molecular Metabolism.2020; 39: 101024. CrossRef - A glycosylated Fc‐fused glucagon‐like peptide‐1 receptor agonist exhibits equivalent glucose lowering to but fewer gastrointestinal side effects than dulaglutide
In Bok An, Mi Sun Byun, Sang In Yang, Yuri Choi, Jung Won Woo, Hak Chul Jang, Young Chul Sung
Diabetes, Obesity and Metabolism.2020; 22(8): 1455. CrossRef - Glucagon-Like Peptide-1 Receptor Agonists in Adult Patients With Type 2 Diabetes: Review of Cardiovascular Outcome Trials
Elodie M. Varin, Brent A. McLean, Julie A. Lovshin
Canadian Journal of Diabetes.2020; 44(1): 68. CrossRef - Cardiovascular outcomes trials with incretin-based medications: a critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors
Alexandros Sachinidis, Dragana Nikolic, Anca Pantea Stoian, Nikolaos Papanas, Omer Tarar, Ali A. Rizvi, Manfredi Rizzo
Metabolism.2020; 111: 154343. CrossRef - GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1β-induced metabolic disturbance and mitochondrial dysfunction
Lili Zhang, Jiali Tian, Sujuan Diao, Guowei Zhang, Mochao Xiao, Dong Chang
Chemico-Biological Interactions.2020; 332: 109252. CrossRef - Predictors of Effectiveness of Glucagon-Like Peptide-1 Receptor Agonist Therapy in Patients with Type 2 Diabetes and Obesity
Alina Yu. Babenko, Daria A. Savitskaya, Yulia A. Kononova, Aleksandra Yu. Trofimova, Anna V. Simanenkova, Elena Yu. Vasilyeva, Evgeny V. Shlyakhto
Journal of Diabetes Research.2019; 2019: 1. CrossRef - Predictors of effectiveness of glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes and obesity
Ekaterina V. Tikhonenko, Alina Y. Babenko, Evgeny V. Shlyakhto
Obesity and metabolism.2019; 15(4): 22. CrossRef - Asian Subpopulations May Exhibit Greater Cardiovascular Benefit from Long-Acting Glucagon-Like Peptide 1 Receptor Agonists: A Meta-Analysis of Cardiovascular Outcome Trials
Yu Mi Kang, Yun Kyung Cho, Jiwoo Lee, Seung Eun Lee, Woo Je Lee, Joong-Yeol Park, Ye-Jee Kim, Chang Hee Jung, Michael A. Nauck
Diabetes & Metabolism Journal.2019; 43(4): 410. CrossRef - Diabetes, Incretin Therapy and Thoracic Aortic Aneurysm – What Does the Evidence Show?
Camilla Krizhanovskii , Anders Franco-Cereceda
Current Vascular Pharmacology.2019; 17(5): 432. CrossRef - Cardiovascular Effects of Different GLP-1 Receptor Agonists in Patients with Type 2 Diabetes
Gül Bahtiyar, Jean Pujals-Kury, Alan Sacerdote
Current Diabetes Reports.2018;[Epub] CrossRef - Efficacy From Strange Sources
Lawrence J. Lesko
Clinical Pharmacology & Therapeutics.2018; 103(2): 253. CrossRef - Exogenous SERP1 attenuates restenosis by restoring GLP-1 receptor activity in diabetic rats following vascular injury
Lishuai Feng, Jianbo Wang, Xu Ma
Biomedicine & Pharmacotherapy.2018; 103: 290. CrossRef - Exenatide exhibits anti‐inflammatory properties and modulates endothelial response to tumor necrosis factor α‐mediated activation
Wojciech Garczorz, Enrique Gallego‐Colon, Agnieszka Kosowska, Agnieszka Kłych‐Ratuszny, Michał Woźniak, Wiesław Marcol, K.J. Niesner, Tomasz Francuz
Cardiovascular Therapeutics.2018;[Epub] CrossRef - Molecular and clinical roles of incretin-based drugs in patients with heart failure
Bassant Orabi, Rasha Kaddoura, Amr S. Omar, Cornelia Carr, Abdulaziz Alkhulaifi
Heart Failure Reviews.2018; 23(3): 363. CrossRef - The effects of Exendin-4 on bone marrow-derived mesenchymal cells
Paola Luciani, Benedetta Fibbi, Benedetta Mazzanti, Cristiana Deledda, Lara Ballerini, Alessandra Aldinucci, Susanna Benvenuti, Riccardo Saccardi, Alessandro Peri
Endocrine.2018; 60(3): 423. CrossRef - Real-world clinical experience of Xultophy in the management of patients with type 2 diabetes in a secondary care clinic
David M. Williams, Natasha Shrikrishnapalasuriyar, Waheeba Syed, Win L. Yin, Richard Chudleigh, Stephen C. Bain, David E. Price, Jeffrey W. Stephens
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2018; 12(6): 1079. CrossRef - Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome
Maja Ðanić, Bojan Stanimirov, Nebojša Pavlović, Svetlana Goločorbin-Kon, Hani Al-Salami, Karmen Stankov, Momir Mikov
Frontiers in Pharmacology.2018;[Epub] CrossRef - Cardiovascular Outcome Trials of Diabetes and Obesity Drugs: Implications for Conditional Approval and Early Phase Clinical Development
Andrew J. Krentz, Gerardo Rodriguez-Araujo
Pharmaceutical Medicine.2017; 31(6): 399. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - GLP-1R as a Target for the Treatment of Diabetic Retinopathy: Friend or Foe?
Rafael Simó, Cristina Hernández
Diabetes.2017; 66(6): 1453. CrossRef - GLP-1 receptor agonists and heart failure in diabetes
André J. Scheen
Diabetes & Metabolism.2017; 43: 2S13. CrossRef - Effects of Incretin-Based Therapies on Diabetic Microvascular Complications
Yu Mi Kang, Chang Hee Jung
Endocrinology and Metabolism.2017; 32(3): 316. CrossRef - Historique des études cardiovasculaires : de l’UGDP… aux dernières études
A.-J. Scheen
Médecine des Maladies Métaboliques.2017; 11: 2S15. CrossRef - Cardiovascular safety and benefits of GLP-1 receptor agonists
Niels B. Dalsgaard, Andreas Brønden, Tina Vilsbøll, Filip K. Knop
Expert Opinion on Drug Safety.2017; 16(3): 351. CrossRef
- Cardioprotective Actions of a Glucagon‐like Peptide‐1 Receptor Agonist on Hearts Donated After Circulatory Death

- Clinical Study
- Glucose-Dependent Insulinotropic Peptide Level Is Associated with the Development of Type 2 Diabetes Mellitus
- Sunghwan Suh, Mi Yeon Kim, Soo Kyoung Kim, Kyu Yeon Hur, Mi Kyoung Park, Duk Kyu Kim, Nam H. Cho, Moon-Kyu Lee
- Endocrinol Metab. 2016;31(1):134-141. Published online March 16, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.1.134
- 3,799 View
- 44 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Incretin hormone levels as a predictor of type 2 diabetes mellitus have not been fully investigated. Therefore, we measured incretin hormone levels to examine the relationship between circulating incretin hormones, diabetes, and future diabetes development in this study.
Methods A nested case-control study was conducted in a Korean cohort. The study included the following two groups: the control group (
n =149), the incident diabetes group (n =65). Fasting total glucagon-like peptide-1 (GLP-1) and total glucose-dependent insulinotropic peptide (GIP) levels were measured and compared between these groups.Results Fasting total GIP levels were higher in the incident diabetes group than in the control group (32.64±22.68 pmol/L vs. 25.54±18.37 pmol/L,
P =0.034). There was no statistically significant difference in fasting total GLP-1 levels between groups (1.14±1.43 pmol/L vs. 1.39±2.13 pmol/L,P =0.199). In multivariate analysis, fasting total GIP levels were associated with an increased risk of diabetes (odds ratio, 1.005;P =0.012) independent of other risk factors.Conclusion Fasting total GIP levels may be a risk factor for the development of type 2 diabetes mellitus. This association persisted even after adjusting for other metabolic parameters such as elevated fasting glucose, hemoglobin A1c, and obesity in the pre-diabetic period.
-
Citations
Citations to this article as recorded by- Mendelian randomization analyses suggest a causal role for circulating GIP and IL-1RA levels in homeostatic model assessment-derived measures of β-cell function and insulin sensitivity in Africans without type 2 diabetes
Karlijn A. C. Meeks, Amy R. Bentley, Themistocles L. Assimes, Nora Franceschini, Adebowale A. Adeyemo, Charles N. Rotimi, Ayo P. Doumatey
Genome Medicine.2023;[Epub] CrossRef - Glucose- and Bile Acid-Stimulated Secretion of Gut Hormones in the Isolated Perfused Intestine Is Not Impaired in Diet-Induced Obese Mice
Jenna E. Hunt, Jens J. Holst, Sara L. Jepsen
Frontiers in Endocrinology.2022;[Epub] CrossRef - Combined treatment with a gastric inhibitory polypeptide receptor antagonist and a peptidyl peptidase-4 inhibitor improves metabolic abnormalities in diabetic mice
Fei Yang, Shan Dang, Hongjun LV, Bingyin Shi
Journal of International Medical Research.2021; 49(1): 030006052098566. CrossRef - Elevated levels of fasting serum GIP may be protective factors for diabetic retinopathy in type 2 diabetes mellitus
LingHong Huang, JingXiong Zhou, Bo Liang, HuiBin Huang, LiangYi Li
International Journal of Diabetes in Developing Countries.2021; 41(4): 543. CrossRef - Enteroendocrine K and L cells in healthy and type 2 diabetic individuals
Tina Jorsal, Nicolai A. Rhee, Jens Pedersen, Camilla D. Wahlgren, Brynjulf Mortensen, Sara L. Jepsen, Jacob Jelsing, Louise S. Dalbøge, Peter Vilmann, Hazem Hassan, Jakob W. Hendel, Steen S. Poulsen, Jens J. Holst, Tina Vilsbøll, Filip K. Knop
Diabetologia.2018; 61(2): 284. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- Mendelian randomization analyses suggest a causal role for circulating GIP and IL-1RA levels in homeostatic model assessment-derived measures of β-cell function and insulin sensitivity in Africans without type 2 diabetes

- Bone Metabolism
- Expression of Glucagon-Like Peptide 1 Receptor during Osteogenic Differentiation of Adipose-Derived Stem Cells
- Yun Kyung Jeon, Min Jung Bae, Ju In Kim, Joo Hyoung Kim, Soo Jong Choi, Su Kyoung Kwon, Joon Hyop An, Sang Soo Kim, Bo Hyun Kim, Yong Ki Kim, In Joo Kim
- Endocrinol Metab. 2014;29(4):567-573. Published online December 29, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.4.567
- 4,326 View
- 35 Download
- 29 Web of Science
- 24 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Glucagon-like peptide 1 (GLP-1), an incretin hormone well known for its glucose-lowering effect, was recently reported to exert an anabolic effect on bone. Although the exact mechanism is not known, it likely involves the GLP-1 receptor (GLP-1R), which is expressed in some osteoblastic cell lines. Adipose-derived stem cells (ADSCs) have mesenchymal stem cell-specific characteristics, including osteoblastic differentiation potential. We evaluated the expression of GLP-1R during osteogenic differentiation of ADSCs.
Methods ADSCs were isolated from subcutaneous adipose tissue obtained from three male donors during plastic surgery and were subjected to osteogenic induction. Mineralization was assessed by Alizarin Red staining on day 21. Expression of alkaline phosphatase (ALP), osteocalcin (OC), and GLP-1R was measured by real-time polymerase chain reaction in triplicate for each patient on days 0, 7, 14, and 21. Target mRNA expression levels were normalized to that of β-actin.
Results ADSCs were fibroblast-like in morphology, adhered to plastic, and had multipotent differentiation potential, as assessed using specific antigen markers. The osteogenic markers ALP and OC were notably upregulated at 21 days. Osteogenic differentiation resulted in a time-dependent increase in the expression of GLP-1R (
P =0.013).Conclusion We demonstrated upregulation of GLP-1R gene expression during osteogenic differentiation of ADSCs. This finding suggests that GLP-1 may induce osteogenic differentiation in bone tissue.
-
Citations
Citations to this article as recorded by- Glucagon-like peptide-1 receptor promotes osteoblast differentiation of dental pulp stem cells and bone formation in a zebrafish scale regeneration model
Shafei Zhai, Changkui Liu, Selvaraj Vimalraj, Raghunandhakumar Subramanian, Shahabe Saquib abullais, Suraj Arora, Sekaran Saravanan
Peptides.2023; 163: 170974. CrossRef - The associations of gut microbiota, endocrine system and bone metabolism
Ye Tu, Xinyi Kuang, Ling Zhang, Xin Xu
Frontiers in Microbiology.2023;[Epub] CrossRef - Effect of Liraglutide on Osteoporosis in a Rat Model of Type 2 Diabetes Mellitus: A Histological, Immunohistochemical, and Biochemical Study
Maha Abdelhamid Fathy, Amal Anbaig, Raja Aljafil, Sherein F El-Sayed, Hanim Magdy Abdelnour, Mona Mostafa Ahmed, Eman M A Abdelghany, Sulaiman Mohammed Alnasser, Shaimaa Mohamed Abdelfattah Hassan, Amany Mohamed Shalaby
Microscopy and Microanalysis.2023; 29(6): 2053. CrossRef - Metabolic responses and benefits of glucagon‐like peptide‐1 (GLP‐1) receptor ligands
Neil Tanday, Peter R. Flatt, Nigel Irwin
British Journal of Pharmacology.2022; 179(4): 526. CrossRef - Exendin‐4 enhances osteogenic differentiation of adipose tissue mesenchymal stem cells through the receptor activator of nuclear factor‐kappa B and osteoprotegerin signaling pathway
Sarah A. Habib, Mohamed M. Kamal, Shohda A. El‐Maraghy, Mahmoud A. Senousy
Journal of Cellular Biochemistry.2022; 123(5): 906. CrossRef - Risk of fracture caused by anti-diabetic drugs in individuals with type 2 diabetes: A network meta-analysis
Wen-Hsuan Tsai, Siang-Ke Kong, Chu-Lin Lin, Kai-Hsuan Cheng, Yi-Ting Cheng, Ming-Nan Chien, Chun-Chuan Lee, Ming-Chieh Tsai
Diabetes Research and Clinical Practice.2022; 192: 110082. CrossRef - Comprehensive Analysis of Novel Genes and Pathways Associated with Osteogenic Differentiation of Adipose Stem Cells
Qiuni Gao, Xiaorong Ma, Zuoliang Qi, Jianxin Shi
Disease Markers.2022; 2022: 1. CrossRef - Novel Insights into the Roles and Mechanisms of GLP-1 Receptor Agonists against Aging-Related Diseases
Wei Peng, Rui Zhou, Ze-Fang Sun, Jia-Wei Long, Yong-Qiang Gong
Aging and disease.2022; 13(2): 468. CrossRef - Correlation of Osteoporosis in Patients With Newly Diagnosed Type 2 Diabetes: A Retrospective Study in Chinese Population
Yuhua Wen, Huijuan Li, Xiaoya Zhang, Peipei Liu, Jing Ma, Liya Zhang, Keqin Zhang, Lige Song
Frontiers in Endocrinology.2021;[Epub] CrossRef - Effects of Incretin-Related Diabetes Drugs on Bone Formation and Bone Resorption
Hideki Kitaura, Saika Ogawa, Fumitoshi Ohori, Takahiro Noguchi, Aseel Marahleh, Yasuhiko Nara, Adya Pramusita, Ria Kinjo, Jinghan Ma, Kayoko Kanou, Itaru Mizoguchi
International Journal of Molecular Sciences.2021; 22(12): 6578. CrossRef - Liraglutide regulates bone destruction and exhibits anti-inflammatory effects in periodontitis in vitro and in vivo
Yunxia Zhang, Xuemin Yuan, Yuyan Wu, Minyu Pei, Man Yang, Xuanye Wu, Yunqing Pang, Jing Wang
Journal of Dentistry.2020; 94: 103310. CrossRef - Exendin-4 regulates Wnt and NF-κB signaling in lipopolysaccharide-induced human periodontal ligament stem cells to promote osteogenic differentiation
Honghong Liu, Jiawen Zheng, Taijing Zheng, Ping Wang
International Immunopharmacology.2019; 75: 105801. CrossRef - Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes
Liga Saulite, Kaspars Jekabsons, Maris Klavins, Ruta Muceniece, Una Riekstina
Phytomedicine.2019; 53: 86. CrossRef - Exendin-4 relieves the inhibitory effects of high glucose on the proliferation and osteoblastic differentiation of periodontal ligament stem cells
Zijun Guo, Rui Chen, Fujun Zhang, Ming Ding, Ping Wang
Archives of Oral Biology.2018; 91: 9. CrossRef - Effects of gastric inhibitory polypeptide, glucagon‐like peptide‐1 and glucagon‐like peptide‐1 receptor agonists on Bone Cell Metabolism
Morten S. S. Hansen, Michaela Tencerova, Jacob Frølich, Moustapha Kassem, Morten Frost
Basic & Clinical Pharmacology & Toxicology.2018; 122(1): 25. CrossRef - Liraglutide, a glucagon-like peptide-1 receptor agonist, facilitates osteogenic proliferation and differentiation in MC3T3-E1 cells through phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), extracellular signal-related kinase (ERK)1/2, and cAMP/pro
Xuelun Wu, Shilun Li, Peng Xue, Yukun Li
Experimental Cell Research.2017; 360(2): 281. CrossRef - The Impact of Glucagon-Like Peptide-1 on Bone Metabolism and Its Possible Mechanisms
Chenhe Zhao, Jing Liang, Yinqiu Yang, Mingxiang Yu, Xinhua Qu
Frontiers in Endocrinology.2017;[Epub] CrossRef - Pathophysiology of Bone Fragility in Patients with Diabetes
Andrea Palermo, Luca D’Onofrio, Raffaella Buzzetti, Silvia Manfrini, Nicola Napoli
Calcified Tissue International.2017; 100(2): 122. CrossRef - Activation of GLP-1 Receptor Promotes Bone Marrow Stromal Cell Osteogenic Differentiation through β-Catenin
Jingru Meng, Xue Ma, Ning Wang, Min Jia, Long Bi, Yunying Wang, Mingkai Li, Huinan Zhang, Xiaoyan Xue, Zheng Hou, Ying Zhou, Zhibin Yu, Gonghao He, Xiaoxing Luo
Stem Cell Reports.2016; 6(4): 579. CrossRef - Mechanisms for the cardiovascular effects of glucagon-like peptide-1
H. Poudyal
Acta Physiologica.2016; 216(3): 277. CrossRef - Perspectives in GLP-1 Research: New Targets, New Receptors
Giulia Cantini, Edoardo Mannucci, Michaela Luconi
Trends in Endocrinology & Metabolism.2016; 27(6): 427. CrossRef - Chronic administration of Glucagon-like peptide-1 receptor agonists improves trabecular bone mass and architecture in ovariectomised mice
M. Pereira, J. Jeyabalan, C.S. Jørgensen, M. Hopkinson, A. Al-Jazzar, J.P. Roux, P. Chavassieux, I.R. Orriss, M.E. Cleasby, C. Chenu
Bone.2015; 81: 459. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef - Impact of Anti-hyperglycemic Medications on Bone Health
Naim M. Maalouf
Clinical Reviews in Bone and Mineral Metabolism.2015; 13(1): 43. CrossRef
- Glucagon-like peptide-1 receptor promotes osteoblast differentiation of dental pulp stem cells and bone formation in a zebrafish scale regeneration model

- Obesity and Metabolism
- A Novel Long-Acting Glucagon-Like Peptide-1 Agonist with Improved Efficacy in Insulin Secretion and β-Cell Growth
- Hee Young Kim, Jong-Ik Hwang, Mi Jin Moon, Jae Young Seong
- Endocrinol Metab. 2014;29(3):320-327. Published online September 25, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.3.320
- 4,286 View
- 49 Download
- 10 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Glucagon-like peptide-1 (GLP-1) is an incretin hormone produced by cleavage of proglucagon in intestinal L-cells. In the pancreas, GLP-1 stimulates post-prandial insulin secretion, promotes insulin biosynthesis, and improves insulin sensitivity. Because of its insulinotropic activity, GLP-1 has been considered a good candidate drug for treatment of diabetes mellitus. However, clinical use of GLP-1 has been limited by its short half-life, as a result of rapid degradation by dipeptidyl peptidase-IV (DPP-IV).
Methods We designed a novel GLP-1 analog,
Xenopus GLP-1 (xGLP)-E4. The Ala residue in the second position of xGLP was replaced with a Ser residue to increase the half-life in the body. The C-terminal tail of exendin-4 was added to enhance the binding affinity for the GLP-1 receptor (GLP1R). The potency of GLP-1 and its analogs was determined by luciferase assay. The stability of GLP1R agonists was evaluated by determining the activity of agonists that had been preincubated in the presence of fetal bovine serum, which contains innate DPP-IV activity. The effects of xGLP-E4 on insulin secretion and β-cell growth were investigated using insulin enzyme-linked immunosorbent assay and cell counting.Results xGLP-E4 exhibited improved stability against DPP-IV activity and increased potency to GLP1R, compared with GLP-1. An increase in glucose-dependent insulin secretion was observed in xGLP-E4-treated pancreatic β-cells. The effect of xGLP-E4 on β-cell growth was greater than that of GLP-1.
Conclusion We developed a novel GLP-1 analog, xGLP-E4, that shows prolonged longevity and improved efficacy. This analog is a potential candidate for treatment of type 2 diabetes.
-
Citations
Citations to this article as recorded by- Novel GLP-1(28–36) amide-derived hybrid peptide A3 with weight loss and hypoglycemic activities
Chen Wang, Binbin Gong, Qianqian Zhu, Jing Han, Lidan Sun
European Journal of Pharmacology.2023; 961: 176200. CrossRef - Intranasal Delivery of a Methyllanthionine-Stabilized Galanin Receptor-2-Selective Agonist Reduces Acute Food Intake
Anneke Kuipers, Márta Balaskó, Erika Pétervári, Andreas Koller, Susanne M. Brunner, Gert N. Moll, Barbara Kofler
Neurotherapeutics.2021; 18(4): 2737. CrossRef - Low-Dose Decitabine Assists Human Umbilical Cord-Derived Mesenchymal Stem Cells in ProtectingβCells via the Modulation of the Macrophage Phenotype in Type 2 Diabetic Mice
Jing Xue, Yu Cheng, Haojie Hao, Jieqing Gao, Yaqi Yin, Songyan Yu, Junyan Zou, Jiejie Liu, Qi Zhang, Yiming Mu
Stem Cells International.2020; 2020: 1. CrossRef - DR10601, a novel recombinant long-acting dual glucagon-like peptide-1 and glucagon receptor agonist for the treatment of obesity and type 2 diabetes mellitus
W. Wang, X. Wen, W. Duan, X. Wang, Y. Chen, J. Dong, Z. Yang, J. Fang, Z. Zhou, G. Yao, Y. Fang, Y. Huang
Journal of Endocrinological Investigation.2020; 43(5): 653. CrossRef - Evolutionary and Comparative Genomics to Drive Rational Drug Design, with Particular Focus on Neuropeptide Seven-Transmembrane Receptors
Michael Furlong, Jae Young Seong
Biomolecules & Therapeutics.2017; 25(1): 57. CrossRef - Comparison of exenatide and acarbose on intra-abdominal fat content in patients with obesity and type-2 diabetes: A randomized controlled trial
Li Shi, Jing Zhu, Ping Yang, Xiaoqiang Tang, Wenlong Yu, Changjie Pan, Moyu Shen, Dalong Zhu, Jinluo Cheng, Xinhua Ye
Obesity Research & Clinical Practice.2017; 11(5): 607. CrossRef - Mesenchymal stem cell therapy in type 2 diabetes mellitus
Li Zang, Haojie Hao, Jiejie Liu, Yijun Li, Weidong Han, Yiming Mu
Diabetology & Metabolic Syndrome.2017;[Epub] CrossRef - Exendin-4 Inhibits Hepatic Lipogenesis by Increasing β-Catenin Signaling
Mi Hae Seo, Jinmi Lee, Seok-Woo Hong, Eun-Jung Rhee, Se Eun Park, Cheol Young Park, Ki Won Oh, Sung Woo Park, Won-Young Lee, Catherine Mounier
PLOS ONE.2016; 11(12): e0166913. CrossRef - Development of Spexin-based Human Galanin Receptor Type II-Specific Agonists with Increased Stability in Serum and Anxiolytic Effect in Mice
Arfaxad Reyes-Alcaraz, Yoo-Na Lee, Gi Hoon Son, Nam Hoon Kim, Dong-Kyu Kim, Seongsik Yun, Dong-Hoon Kim, Jong-Ik Hwang, Jae Young Seong
Scientific Reports.2016;[Epub] CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef - Biased signalling: the instinctive skill of the cell in the selection of appropriate signalling pathways
Ying Liu, Yang Yang, Richard Ward, Su An, Xiao-Xi Guo, Wei Li, Tian-Rui Xu
Biochemical Journal.2015; 470(2): 155. CrossRef
- Novel GLP-1(28–36) amide-derived hybrid peptide A3 with weight loss and hypoglycemic activities

- Obesity and Metabolism
- Clinical Application of Glucagon-Like Peptide 1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus
- Young Min Cho, Rhonda D. Wideman, Timothy J. Kieffer
- Endocrinol Metab. 2013;28(4):262-274. Published online December 12, 2013
- DOI: https://doi.org/10.3803/EnM.2013.28.4.262
- 5,291 View
- 82 Download
- 37 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Glucagon-like peptide 1 (GLP-1) is secreted from enteroendocrine L-cells in response to oral nutrient intake and elicits glucose-stimulated insulin secretion while suppressing glucagon secretion. It also slows gastric emptying, which contributes to decreased postprandial glycemic excursions. In the 1990s, chronic subcutaneous infusion of GLP-1 was found to lower blood glucose levels in patients with type 2 diabetes. However, GLP-1's very short half-life, arising from cleavage by the enzyme dipeptidyl peptidase 4 (DPP-4) and glomerular filtration by the kidneys, presented challenges for clinical use. Hence, DPP-4 inhibitors were developed, as well as several GLP-1 analogs engineered to circumvent DPP-4-mediated breakdown and/or rapid renal elimination. Three categories of GLP-1 analogs, are being developed and/or are in clinical use: short-acting, long-acting, and prolonged-acting GLP-1 analogs. Each class has different plasma half-lives, molecular size, and homology to native GLP-1, and consequently different characteristic effects on glucose metabolism. In this article, we review current clinical data derived from each class of GLP-1 analogs, and consider the clinical effects reported for each category in recent head to head comparison studies. Given the relatively brief clinical history of these compounds, we also highlight several important efficacy and safety issues which will require further investigation.
-
Citations
Citations to this article as recorded by- The efficacy and safety of combined GLP-1RA and basal insulin therapy among inadequately controlled T2D with premixed insulin therapy
Jhih-Syuan Liu, Sheng-Chiang Su, Feng-Chih Kuo, Peng-Fei Li, Chia-Luen Huang, Li-Ju Ho, Kuan-Chan Chen, Yi-Chen Liu, Chih-Ping Lin, An-Che Cheng, Chien-Hsing Lee, Fu-Huang Lin, Yi-Jen Hung, Hsin-Ya Liu, Chieh-Hua Lu, Chang-Hsun Hsieh
Medicine.2023; 102(10): e33167. CrossRef - Pharmacogenetics of Glucagon-like-peptide-1 receptor in diabetes management
Mariya Kalinkova, Tanya Kadiyska, Teodora Handjieva-Darlenska
Pharmacia.2023; 70(2): 383. CrossRef - Anti-inflammatory potential of liraglutide, a glucagon-like peptide-1 receptor agonist, in rats with peripheral acute inflammation
Irem Mert, Ayhan Cetinkaya, Mujgan Gurler, Canan Akünal Turel, Humeyra Celik, Ibrahim Ethem Torun, Idris Turel
Inflammopharmacology.2022; 30(3): 1093. CrossRef - Exenatide improves hepatocyte insulin resistance induced by different regional adipose tissue
Chuanmin Bai, Yujun Wang, Zhi Niu, Yaxin Guan, Jingshan Huang, Xin Nian, Fan Zuo, Juan Zhao, Tsutomu Kazumi, Bin Wu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Peptidyl and Non-Peptidyl Oral Glucagon-Like Peptide-1 Receptor Agonists
Hun Jee Choe, Young Min Cho
Endocrinology and Metabolism.2021; 36(1): 22. CrossRef - Glucagon-like Peptide-1 Improves Fatty Liver and Enhances Thermogenesis in Brown Adipose Tissue via Inhibiting BMP4-Related Signaling Pathway in High-Fat-Diet-Induced Obese Mice
Xingchun Wang, Bingwei Ma, Jiaqi Chen, Hui You, Chunjun Sheng, Peng Yang, Shen Qu, Vito Angelo Giagulli
International Journal of Endocrinology.2021; 2021: 1. CrossRef - Exenatide ameliorates experimental non-alcoholic fatty liver in rats via suppression of toll-like receptor 4/NFκB signaling: Comparison to metformin
Zeinab A. Saad, Dina M. Khodeer, Sawsan A. Zaitone, Amal A.M. Ahmed, Yasser M. Moustafa
Life Sciences.2020; 253: 117725. CrossRef - Exenatide ameliorates hepatic steatosis and attenuates fat mass and FTO gene expression through PI3K signaling pathway in nonalcoholic fatty liver disease
Shan Li, Xiaoman Wang, Jielei Zhang, Jingyi Li, Xiaogang Liu, Yuanyuan Ma, Chao Han, Lixia Zhang, Lili Zheng
Brazilian Journal of Medical and Biological Research.2018;[Epub] CrossRef - Efficacy and safety of combination therapy with an α‐glucosidase inhibitor and a dipeptidyl peptidase‐4 inhibitor in patients with type 2 diabetes mellitus: A systematic review with meta‐analysis
Se Hee Min, Jeong‐Hwa Yoon, Seokyung Hahn, Young Min Cho
Journal of Diabetes Investigation.2018; 9(4): 893. CrossRef - Glucagon-like peptide-1 affects human umbilical vein endothelial cells in high glucose by the PI3K/Akt/eNOS signaling pathway
Jie Wu, Pingfan Guo, Tianmin He, Fanggang Cai
Turkish Journal of Biochemistry.2018; 43(2): 119. CrossRef - What next after basal insulin? Treatment intensification with lixisenatide in Asian patients with type 2 diabetes mellitus
Wing B. Chan, Andrea Luk, Wing S. Chow, Vincent T.F. Yeung
Journal of Diabetes.2017; 9(6): 562. CrossRef - Treatment potential of the GLP-1 receptor agonists in type 2 diabetes mellitus: a review
L. Østergaard, Christian S. Frandsen, S. Madsbad
Expert Review of Clinical Pharmacology.2016; 9(2): 241. CrossRef - Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program
Johan Jendle, George Grunberger, Thomas Blevins, Francesco Giorgino, Ryan T. Hietpas, Fady T. Botros
Diabetes/Metabolism Research and Reviews.2016; 32(8): 776. CrossRef - Glucagon‐like peptide‐1(GLP‐1) receptor agonists: potential to reduce fracture risk in diabetic patients?
Guojing Luo, Hong Liu, Hongyun Lu
British Journal of Clinical Pharmacology.2016; 81(1): 78. CrossRef - Impact of postprandial glucose control on diabetes-related complications: How is the evidence evolving?
Sten Madsbad
Journal of Diabetes and its Complications.2016; 30(2): 374. CrossRef - Risk assessment and management of post-transplant diabetes mellitus
Eugene Han, Myoung Soo Kim, Yu Seun Kim, Eun Seok Kang
Metabolism.2016; 65(10): 1559. CrossRef - Recent advances in oral delivery of peptide hormones
Pegah Varamini, Istvan Toth
Expert Opinion on Drug Delivery.2016; 13(4): 507. CrossRef - Exendin-4 Inhibits Hepatic Lipogenesis by Increasing β-Catenin Signaling
Mi Hae Seo, Jinmi Lee, Seok-Woo Hong, Eun-Jung Rhee, Se Eun Park, Cheol Young Park, Ki Won Oh, Sung Woo Park, Won-Young Lee, Catherine Mounier
PLOS ONE.2016; 11(12): e0166913. CrossRef - Review of head‐to‐head comparisons of glucagon‐like peptide‐1 receptor agonists
Sten Madsbad
Diabetes, Obesity and Metabolism.2016; 18(4): 317. CrossRef - Does Bosentan Protect Diabetic Brain Alterations in Rats? The Role of Endothelin‐1 in the Diabetic Brain
Recep Demir, Elif Cadirci, Erol Akpinar, Yasemin Cayir, Hasan Tarik Atmaca, Harun Un, Celalettin Semih Kunak, Muhammed Yayla, Zafer Bayraktutan, Ilknur Demir
Basic & Clinical Pharmacology & Toxicology.2015; 116(3): 236. CrossRef - Efficacy and tolerability of albiglutide versus placebo or pioglitazone over 1 year in people with type 2 diabetes currently taking metformin and glimepiride: HARMONY 5
P. D. Home, P. Shamanna, M. Stewart, F. Yang, M. Miller, C. Perry, M. C. Carr
Diabetes, Obesity and Metabolism.2015; 17(2): 179. CrossRef - Differential effects of prandial and non-prandial GLP-1 receptor agonists in type 2 diabetes therapy
Jaime A. Davidson
Postgraduate Medicine.2015; 127(8): 827. CrossRef - GLP‐1 receptor agonists: effects on the progression of non‐alcoholic fatty liver disease
Jia Liu, Guang Wang, Yumei Jia, Yuan Xu
Diabetes/Metabolism Research and Reviews.2015; 31(4): 329. CrossRef - Efficacy and safety of teneligliptin, a dipeptidyl peptidase‐4 inhibitor, combined with metformin in Korean patients with type 2 diabetes mellitus: a 16‐week, randomized, double‐blind, placebo‐controlled phase III trial
M. K. Kim, E.‐J. Rhee, K. A. Han, A. C. Woo, M.‐K. Lee, B. J. Ku, C. H. Chung, K.‐A. Kim, H. W. Lee, I. B. Park, J. Y. Park, H. C. Chul Jang, K. S. Park, W. I. Jang, B. Y. Cha
Diabetes, Obesity and Metabolism.2015; 17(3): 309. CrossRef - Incretin physiology and pathophysiology from an Asian perspective
Young Min Cho
Journal of Diabetes Investigation.2015; 6(5): 495. CrossRef - Response: Economic Impact of Combining Metformin with Dipeptidyl Peptidase-4 Inhibitors in Diabetic Patients with Renal Impairment in Spanish Patients (Diabetes Metab J2015;39:74-81)
Antoni Sicras-Mainar, Ruth Navarro-Artieda
Diabetes & Metabolism Journal.2015; 39(2): 173. CrossRef - Clinical Application of Glucagon-Like Peptide-1 Receptor Agonists
Se Hee Min, Young Min Cho
The Journal of Korean Diabetes.2015; 16(4): 252. CrossRef - Autophagy deficiency in β cells blunts incretin-induced suppression of glucagon release from α cells
Min Joo Kim, Ok Kyong Choi, Kyung Sil Chae, Hakmo Lee, Sung Soo Chung, Dong-Sik Ham, Ji-Won Kim, Kun-Ho Yoon, Kyong Soo Park, Hye Seung Jung
Islets.2015; 7(5): e1129096. CrossRef - Glucagon-Like Peptide-1 Increases Mitochondrial Biogenesis and Function in INS-1 Rat Insulinoma Cells
Mi Yeon Kang, Tae Jung Oh, Young Min Cho
Endocrinology and Metabolism.2015; 30(2): 216. CrossRef - Interleukin-6 CpG Methylation and Body Weight Correlate Differently in Type 2 Diabetes Patients Compared to Obese and Lean Controls
Eva Aumueller, Marlene Remely, Hanna Baeck, Berit Hippe, Helmut Brath, Alexander G. Haslberger
Lifestyle Genomics.2015; 8(1): 26. CrossRef - The glucagon-like peptide 1 receptor agonist enhances intrinsic peroxisome proliferator-activated receptor γ activity in endothelial cells
Hirohisa Onuma, Kouichi Inukai, Atsuko Kitahara, Rie Moriya, Susumu Nishida, Toshiaki Tanaka, Hidenori Katsuta, Kazuto Takahashi, Yoshikazu Sumitani, Toshio Hosaka, Hitoshi Ishida
Biochemical and Biophysical Research Communications.2014; 451(2): 339. CrossRef - Brief Review of Articles in 'Endocrinology and Metabolism' in 2013
Won-Young Lee
Endocrinology and Metabolism.2014; 29(3): 251. CrossRef - Cytokines and Hormones That Contribute to the Positive Association between Fat and Bone
Dorit Naot, Jillian Cornish
Frontiers in Endocrinology.2014;[Epub] CrossRef - Study of Postprandial Lipaemia in Type 2 Diabetes Mellitus: Exenatide versus Liraglutide
Maria Voukali, Irene Kastrinelli, Sapfo Stragalinou, Dimitra Tasiopoulou, Pinelopi Paraskevopoulou, Nicholas Katsilambros, Alexandros Kokkinos, Nicholas Tentolouris, Ioannis Ioannidis
Journal of Diabetes Research.2014; 2014: 1. CrossRef - The cardiometabolic benefits of glycine: Is glycine an ‘antidote’ to dietary fructose?
Mark F McCarty, James J DiNicolantonio
Open Heart.2014; 1(1): e000103. CrossRef - Nuevos fármacos antidiabéticos: avanzando hacia el control integral de la diabesidad
J.J. Gorgojo-Martínez
Hipertensión y Riesgo Vascular.2014; 31(2): 45. CrossRef - Managing Loss of Glycemic Control in Middle-Aged Patients With Diabetes: The Role of GLP-1 Receptor Agonists in Combination-Therapy Regimens
Thomas B. Repas
Journal of Osteopathic Medicine.2014; 114(s52): 14. CrossRef
- The efficacy and safety of combined GLP-1RA and basal insulin therapy among inadequately controlled T2D with premixed insulin therapy


 KES
KES

 First
First Prev
Prev



