Cardiovascular Outcomes with Finerenone According to Glycemic Status at Baseline and Prior Treatment with Newer Antidiabetics among Patients with Type 2 Diabetes Mellitus
Article information
Abstract
Type 2 diabetes mellitus (T2DM) and cardiovascular disease are closely interconnected. We sought to determine the cardioprotective action of finerenone according to prior treatment with newer antidiabetics and glycemic status. We searched PubMed and Cochrane Library from inception to October 1, 2021 for randomized controlled trials (RCTs) assessing the effect of finerenone on major adverse cardiovascular outcomes in patients with T2DM. We set the primary endpoint as major adverse cardiovascular events (MACE), defined as the composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. We finally included two RCTs in our quantitative synthesis. Compared to placebo, finerenone induced a 23% risk reduction for the composite cardiovascular endpoint, regardless of prior glycemia. We also showed that finerenone provided significant cardiovascular benefit for obese patients with T2DM compared to placebo, although this benefit was diminished for subjects with a body mass index lower than 30 kg/m2. Finally, the combination of finerenone with sodium-glucose co-transporter-2 inhibitors or glucagon-like peptide-1 receptor agonists did not produce a significant risk reduction for MACE. We conclude that finerenone provides significant cardiovascular benefits for patients with T2DM, especially for those who are obese, while glycemic status or treatment with newer antidiabetics at baseline does not affect the observed cardioprotective action.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) and cardiovascular disease are interconnected since the latter represents the major cause of death in people with T2DM [1]. Patients with T2DM are usually overweight or obese and also suffer from significant comorbidities, such as chronic kidney disease (CKD) and heart failure. Despite improvements in early diagnosis and the implementation of appropriate therapeutic strategies, the burden of cardiovascular death for patients with T2DM remains high [2]. Newer classes of antidiabetic drugs—namely, sodium-glucose co-transporter-2 (SGLT-2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1RAs)—have proven cardiovascular efficacy and safety, and are now considered the treatments of choice [3–6]. These drug classes exert multiple, pleiotropic effects; however, the value of adequate glycemic control remains undoubted for the prevention of cardiovascular morbidity and mortality [7].
Finerenone, a nonsteroidal selective mineralocorticoid receptor antagonist, has recently attracted scientific interest due to its cardiovascular and renal benefits observed in patients with T2DM and CKD [8,9]. A number of pre-clinical, experimental studies have addressed the distinct mechanism of action of finerenone and its great potential for the treatment of patients with cardio-renal disease [10]. Based on the cardioprotective and renoprotective action of finerenone [11], a question that inevitably arises is whether the aforementioned drug classes could be combined in clinical practice, and if yes, whether there is a synergistic effect, as seen in some experimental models [12].
Therefore, we sought to determine the cardiovascular efficacy of finerenone in subjects with T2DM according to: (1) glycemic status and body weight at baseline and (2) prior treatment with antidiabetic drug classes with established cardio-renal benefits (i.e., SGLT-2 inhibitors and GLP-1RAs).
METHODS
We searched PubMed and the Cochrane Library from inception to October 1, 2021 for randomized controlled trials (RCTs) assessing the effect of finerenone versus placebo or an active comparator on major adverse cardiovascular outcomes. We implemented in both databases the following search strategy: (finerenone) OR (kerendia) AND (((((myocardial infarction) OR (stroke)) OR (heart failure)) OR (cardiovascular death)) OR (major adverse cardiovascular event)) OR (MACE). We planned to include RCTs enrolling adult patients with T2DM. We also searched gray literature sources (clinicaltrials.gov, Google Scholar, and abstracts retrieved from major medical conferences in the field of cardiovascular medicine or cardiology) for potentially eligible trials not available in the aforementioned databases.
We set as the primary endpoint major adverse cardiovascular events, defined as the composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. We performed subgroup analyses according to glycemic control status based on glycated hemoglobin levels at baseline, prior treatment with newer antidiabetic drug classes (SGLT-2 inhibitors and GLP-1RAs) and finally, obesity status at baseline (yes/no).
Two independent reviewers (D.P. and C.P.) extracted the data of interest from the eligible studies. As we assessed only dichotomous variables, differences were calculated using the risk ratio (RR), with 95% confidence intervals (CIs), after implementation of the Mantel-Haenszel random-effects formula. Statistical heterogeneity among studies was assessed using the I2 statistic. All analyses were performed at the 0.05 significance level with RevMan 5.3 software (https://training.cochrane.org).
Two independent reviewers (D.P. and C.P.) assessed the quality of the included RCTs using the Revised Cochrane risk of bias tool for randomized trials (RoB 2.0) for the primary efficacy outcome [13]. Discrepancies between reviewers were resolved by discussion, consensus, or arbitration by a third senior reviewer (M.D.).
RESULTS
We retrieved 64 results from PubMed and 22 results from Cochrane Library, while we did not identify any further relevant studies through searching the gray literature. After de-duplication, we finally assessed 77 reports for potential inclusion in our analysis. We performed screening at the title and abstract level, reaching a total of two RCTs for inclusion in our quantitative synthesis: the Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD) trial [8] and the post-hoc analysis of the Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD) trial evaluating the effect of finerenone compared to placebo on major adverse cardiovascular outcomes [14]. Both trials included subjects with T2DM and CKD, assigned either to finerenone or placebo. A thorough description of participants’ baseline characteristics of interest is provided in Table 1.
Both the FIDELIO-DKD and FIGARO-DKD trials enrolled subjects with long-standing T2DM, with insufficient glycemic control, who were mostly overweight or obese. The vast majority of participants had concomitant hypertension, while almost half of them had a history of established cardiovascular disease. Almost all subjects used renin-angiotensin-aldosterone system blockers, insulin usage rates ranged from 54.3% to 64.1%, but the studies did not differ substantially regarding the usage rates of other antidiabetic drug classes, such as metformin, sulfonylureas, dipeptidyl-peptidase-4 inhibitors, or alpha-glucosidase inhibitors (Table 1). Therefore, the patient population was considered homogeneous in terms of the baseline characteristics of specific interest.
As shown in Fig. 1, finerenone compared to placebo induced a 23% risk reduction for the composite cardiovascular endpoint (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) regardless of prior glycemic status (glycated hemoglobin greater or lower than 7.5%, as pre-defined in both included RCTs). No subgroup difference was documented for this comparison (P=0.99).
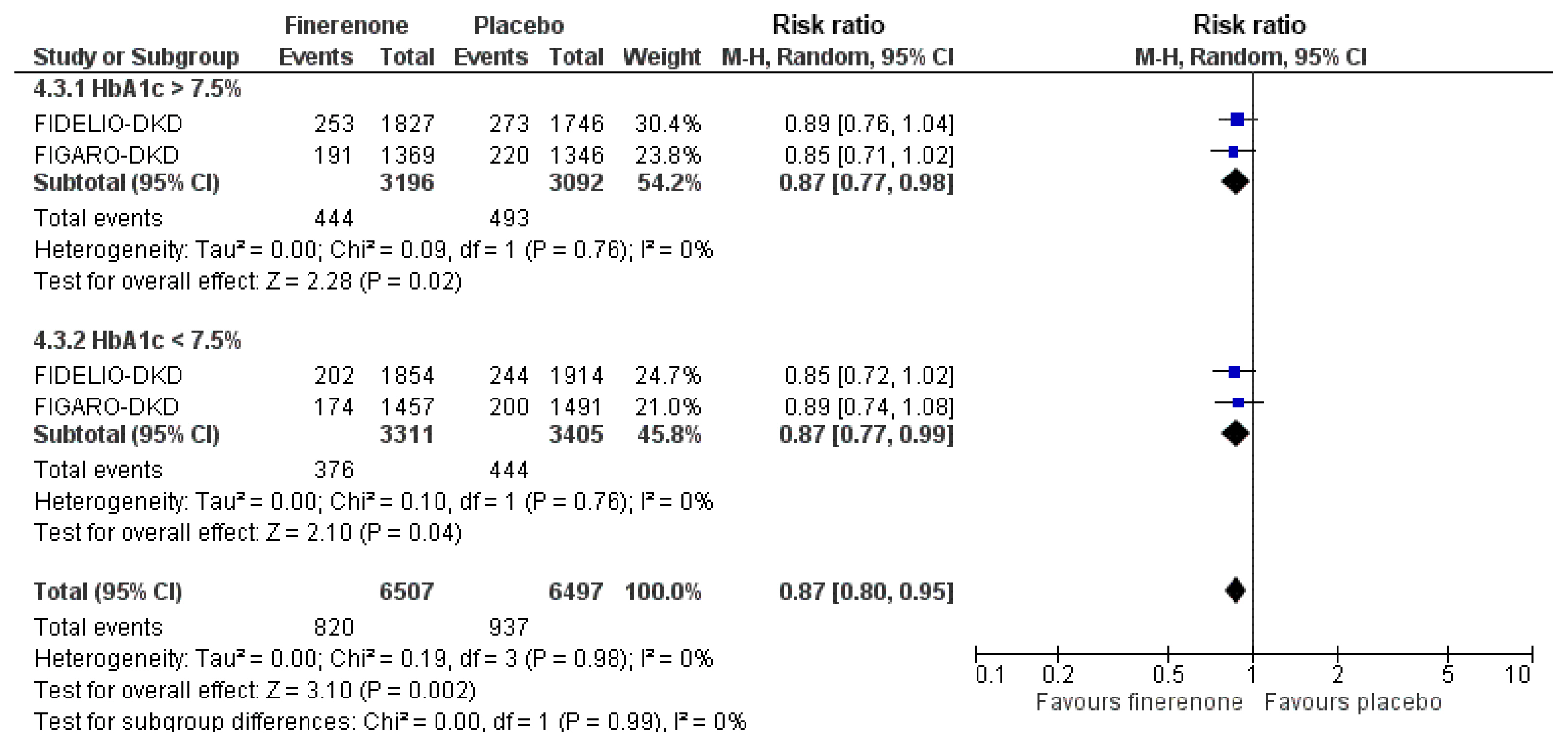
Effect of finerenone compared to placebo on the risk for the cardiovascular composite endpoint according to glycemic status at baseline. M–H, Mantel-Haenszel; CI, confidence interval; HbA1c, glycated hemoglobin; FIDELIO-DKD, Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease; FIGARO-DKD, Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease.
When we analyzed data according to previous treatment with SGLT-2 inhibitors, we observed that the combination of finerenone and an SGLT-2 inhibitor resulted in a non-significant decrease in the risk for the primary composite endpoint (RR, 0.79; 95% CI, 0.46 to 1.34; I2=41%), while finerenone without the addition of an SGLT-2 inhibitor led to a significant decrease in the risk for a major adverse cardiovascular event (RR, 0.88; 95% CI, 0.81 to 0.97; I2=0%), as depicted in Supplemental Fig. S1. Notably, no subgroup difference was identified (P=0.67). In addition, we analyzed data of interest according to prior treatment with GLP-1RAs, and demonstrated that the combination of finerenone with a GLP-1RA did not have a significant effect on the primary cardiovascular endpoint (RR, 0.83; 95% CI, 0.54 to 1.27; I2=39%), while finerenone alone resulted in a significant decrease by 12% compared to placebo (RR, 0.88; 95% CI, 0.81 to 0.97; I2=0%), as shown in Supplemental Fig. S2. The most reasonable explanation for these observations is that both FIGARO-DKD and FIDELIO-DKD were not adequately powered to assess the cardiovascular efficacy of combination of finerenone with newer antidiabetics, as implied by the relatively low usage rates of both drug classes in these two trials.
In addition, we showed that finerenone provided significant cardiovascular benefit for obese patients with T2DM, compared to placebo, which was diminished for subjects with a body mass index lower than 30 kg/m2. However, again, no subgroup difference was demonstrated (P=0.51), as depicted in Supplemental Fig. S3.
Of note, the usage rates of other antidiabetic drug classes, renin-angiotensin-aldosterone system blockers, and diuretics did not significantly differ between the two studies; therefore, they do not seem to have influenced the results. We consider that the major limitation of the present meta-analysis is the limited number of eligible trials for inclusion.
DISCUSSION
Finerenone leads to an early decrease in the estimated glomerular filtration rate (eGFR), resulting in a long-term preservation of the eGFR, along with a significant decrease in albuminuria [15]. Decrease in the intraglomerular pressure along with a decrease in the albumin and metabolic load in proximal tubular renal cells have been suggested as a mechanism underlying its reno-protective effects [15]. Reductions in renal, cardiac, and vascular fibrosis along with amelioration of concomitant inflammation have also been shown with finerenone in experimental models [15,16]. Both SGLT-2 inhibitors and GLP-1RAs have been shown to decrease albuminuria, while they also improve vascular, renal, and cardiac function by suppressing inflammation, fibrosis, oxidative stress, and atherogenesis [17,18]. In addition, both drug classes induce body weight reduction (greater with GLP-1RAs), while SGLT-2 inhibitors produce a substantial decrease in blood pressure, under both static and ambulatory conditions. Therefore, both antidiabetic drug classes exert to some extent effects that are similar to those documented for finerenone. The latter inevitably leads to the question of whether these drug classes could be combined in clinical practice, and if such a combination would provide additive beneficial effects. This research question motivated us to conduct the present analysis.
Despite being promising, this analysis failed to demonstrate additive cardiovascular benefits when finerenone is combined with an antidiabetic with established cardioprotective beneficial effects (either GLP-1RA or an SGLT-2 inhibitor). However, we showed that finerenone provides significant cardiovascular benefits for patients with T2DM, especially for those who are obese, while glycemic status at baseline does not affect the observed cardioprotective action.
The small number of included trials in the present meta-analysis may limit the generalizability of the generated results, which should be interpreted with caution. In the future, sufficiently powered trials addressing the potentially synergistic effects of these combinations will provide answers to those interesting research questions.
Supplementary Information
Effect of finerenone compared to placebo on the risk for the cardiovascular composite endpoint according to prior treatment with sodium-glucose co-transporter-2 (SGLT-2) inhibitors. M–H, Mantel-Haenszel; CI, confidence interval; FIDELIO-DKD, Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease; FIGARO-DKD, Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease.
Effect of finerenone compared to placebo on the risk for the cardiovascular composite endpoint according to prior treatment with glucagon-like peptide-1 receptor agonists (GLP-1RAs). M–H, Mantel-Haenszel; CI, confidence interval; FIDELIO-DKD, Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease; FIGARO-DKD, Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease.
Effect of finerenone compared to placebo on the risk for the cardiovascular composite endpoint according to the presence or absence of obesity at baseline. M–H, Mantel-Haenszel; CI, confidence interval; BMI, body mass index; FIDELIO-DKD, Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease; FIGARO-DKD, Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION
Conception or design: D.P., M.D. Acquisition, analysis, or interpretation of data: D.P., C.P., V.V. Drafting the work or revising: D.P., C.P., A.K., M.D. Final approval of the manuscript: D.P., C.P., A.K., V.V., M.D.

