Recent Insights into Insulin-Like Growth Factor Binding Protein 2 Transcriptional Regulation
Article information
Abstract
Insulin-like growth factor binding proteins (IGFBPs) are major regulators of insulin-like growth factor bioavailability and activity in metabolic signaling. Seven IGFBP family isoforms have been identified. Recent studies have shown that IGFBPs play a pivotal role in metabolic signaling and disease, including the pathogenesis of obesity, diabetes, and cancer. Although many studies have documented the various roles played by IGFBPs, transcriptional regulation of IGFBPs is not well understood. In this review, we focus on the regulatory mechanisms of IGFBP gene expression, and we summarize the findings of transcription factor activity in the IGFBP promoter region.
INTRODUCTION
Insulin-like growth factor binding proteins (IGFBPs) are a superfamily member of homologous proteins present in serum [1]. All members of the IGFBP family include a cysteine-rich domain, which contains the GCGCCXXC motif in the N-terminal and C-terminal domains [2]. Insulin-like growth factors (IGFs) are central hormones involved in metabolic signaling, affecting glucose uptake, lipogenesis, glycogen storage, and suppression of protein degradation [3]. Studies have shown that IGFBPs have IGF-dependent and IGF-independent functions [1]. Within the IGFBP family, IGFBP-2 is a protein encoded by the IGFBP-2 gene [4]. Observations on the identification and function of IGFBP-2 in metabolic signaling and disease are discussed in this review. These data provide new insights into our understanding of the pathophysiology of metabolic syndrome and have important clinical implications, although additional research is required.
TISSUE SURVEY OF IGFBP-2 GENE EXPRESSION
IGFBP-2 is a 36-kDa protein that it is mainly expressed during embryonic development; it is also expressed in adult liver and adipocytes, and in the central nervous and reproductive systems [5]. Tissue survey data have elucidated the IGFBP-2 gene expression profile (Fig. 1). These data show that IGFBP-2 is mainly expressed in the liver, heart, kidney, and prostate.
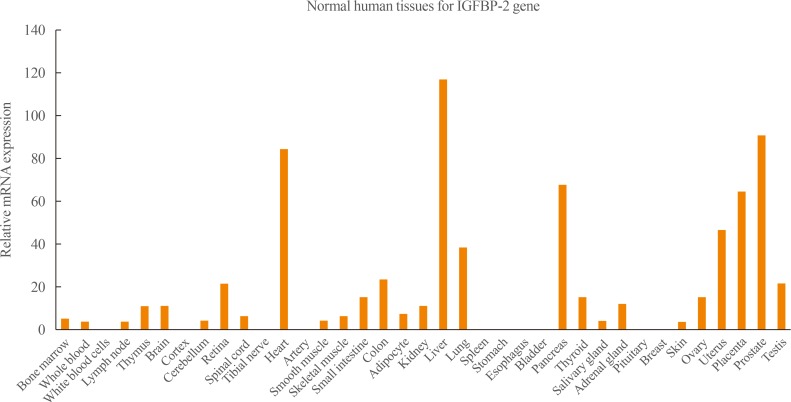
Insulin-like growth factor binding protein 2 (IGFBP-2) expression profile from Genecards database (www.genecards.org).
COMPARISON OF IGFBP-2 GENE SEQUENCES BETWEEN SPECIES
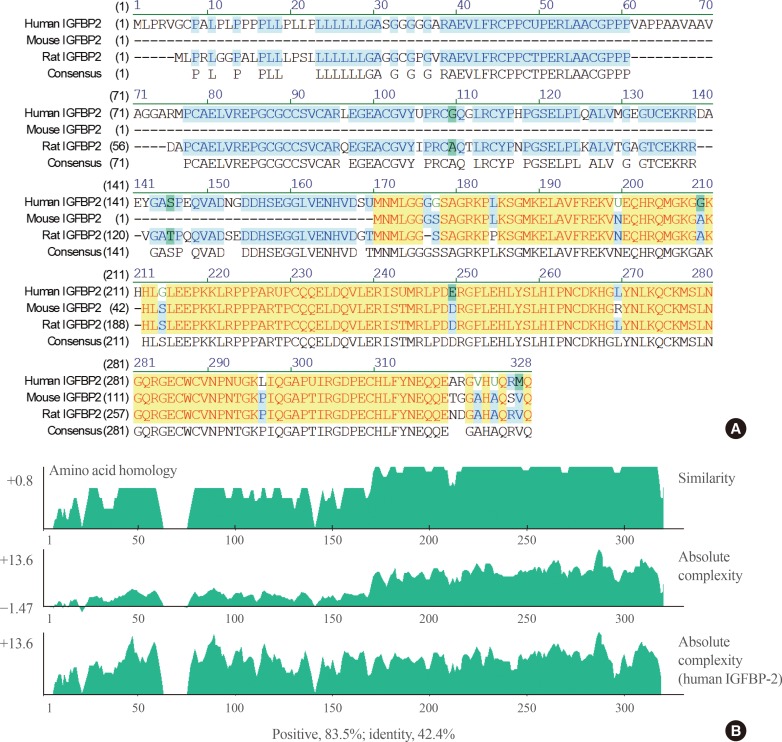
When the IGFBP-2 sequence was compared between species, several regions of high similarity between promoter sequences were observed (Fig. 2). The IGFBP-2 gene is identical in 49.4% of sequences between humans, rats, and mice. Similarly, exon sequences for the IGFBP-2 gene are about 42.2% identical across species (Fig. 3). In particular, consensus sequences for the major transcription factors match significantly between humans, mice, and rats. Moreover, the IGFBP-2 gene C-terminal domain, including the consensus sequence for IGF binding and the CWCV (cysteine-tryptophan-cysteine-valine) domain, is highly conserved across a variety of species [6]. These data indicate that IGFBP-2 may have high binding affinity with IGFs.
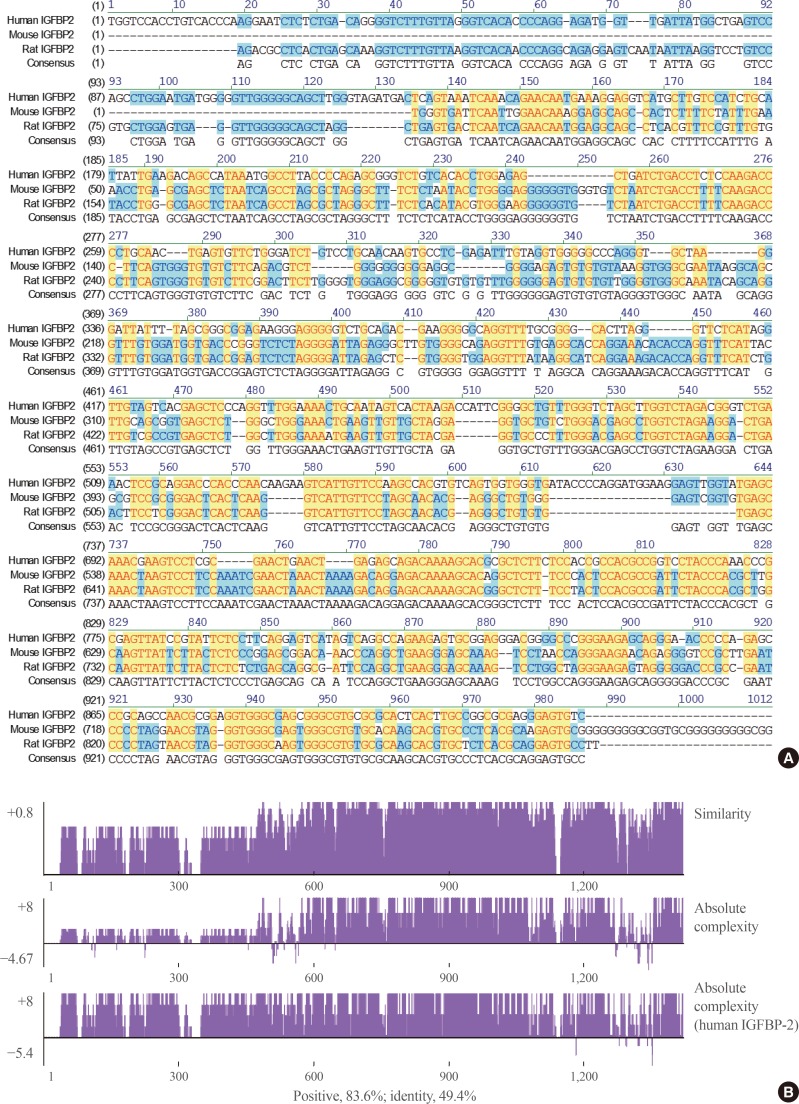
Homology comparison of the insulin-like growth factor binding protein 2 (IGFBP-2) promoter region between species which show highly conserved regions with yellow color. (A) Alignment of human, mouse and rat IGFBP-2 promoter sequences between −1,000 and 1 bp from transcription start site. (B) Similarity plots of aligned IGFBP-2 promoter sequences between species.
TRANSCRIPTIONAL REGULATION OF IGFBP-2
Although the secretion of IGFBP-2 protein is well established in metabolic signaling, the molecular mechanisms of the IGFBP-2 gene have only recently been elucidated. A few transcription factors, including peroxisome proliferation-activator receptor α (PPARα), multiple endocrine neoplasia type 1 (MEN1), CCAAT-enhancer-binding protein α (C/EBPα), and hypoxia-inducible factor 1 (HIF-1), have been identified on the IGFBP-2 promoter (Fig. 4).

Summary of transaction factor binding to the promoter regions of the insulin-like growth factor binding protein 2 (IGFBP-2) gene for human and mouse. PPAR, peroxisome proliferation-activator receptor; PPRE, PPAR response element; C/EBPα, CCAAT-enhancer-binding protein α; RXRα, retinoid X receptor α.
One study found that IGFBP-2 expression increased during prolonged fasting, and the plasma concentration of IGFBP-2 was consistently induced for 48 hours with no change postprandial or after glucose challenge [7]. Increased IGFBP-2 expression during fasting is regulated by PPARα [89]. The PPARα response element (PPRE) was identified on a mouse IGFBP-2 promoter, indicating that IGFBP-2 is a direct target of PPARα. Moreover, mRNA expression of the IGFBP-2 gene is activated by metformin, which is an antidiabetic drug for patients with diabetes and obesity, and the serum level of IGFBP-2 is stimulated in metformin-challenged diabetic patients [10]. A recent study in humans suggested that the circulating concentration of IGFBP-2 is associated with metabolic syndrome, including insulin resistance and obesity [1112]. These results suggest that overexpression of the IGFBP-2 gene plays a protective role against insulin resistance.
Previous studies found that insulin suppresses induction of IGFBP-2, as well as IGFBP-1 [13]. Moreover, hepatic IGFBP-2 expression is decreased by insulin infusion, whereas IGFBP-1 mRNA recovers to baseline within 1 hour postinsulin challenge. These results suggest that IGFBP-2 expression level is very important for modulating insulin and IGF-1 sensitivity. In addition, the expression level of IGFBP-2 could potentially serve as a biomarker of metabolic disease, such as diabetes or insulin resistance. Although IGFBP-2 is mainly expressed in the liver, transcriptional regulation of the IGFBP-2 gene has been studied in other tissues [111415]. In particular, IGFBP-2 gene expression in 3T3-L1 adipocytes is regulated by C/EBPα, even though IGFBP-2 is expressed at a very low level in white adipose tissue [16]. Compared to the liver, insulin stimulated IGFBP-2 gene expression in adipocytes. Insulin activates IGFBP-2 expression in 3T3-L1 adipocytes and MCF-7 (Michigan Cancer Foundation 7) breast cancer cell lines through the phosphoinositide 3-kinase/mechanistic target of rapamycin signaling axis [1617]. These findings indicate that insulin signaling might increase IGFBP-2 secretion by activating insulin-induced, adipocyte-specific expression of the C/EBPα gene in differentiated 3T3-L1 adipocytes.
Transcriptional regulation of the IGFBP-2 gene also occurs in tumorigenesis [8181920]. During tumor growth, IGFBP-2 expression is regulated by the transcription factor HIF-1α in hypoxic conditions [8]. In addition, IGFBP-2 mRNA is upregulated by the tumor suppressor gene p53 in a dose-dependent manner, indicating that IGFBP-2 is a p53 target [20]. Also, the IGFBP-2 gene was upregulated by disruption of the menin gene in an animal model and stable cell lines [2122]. Menin is a nuclear protein-encoded Men1 gene, which is mutated in patients with MEN1 [22]. MEN1 is a syndrome characterized by tumorigenesis in multiple endocrine organs (e.g., pancreas and parathyroids) [22]. Menin is required to repress endogenous IGFBP-2 expression by modifying the chromatin structure surrounding the IGFBP-2 gene promoter. Although IGFBP-2 promoter activity is decreased by overexpression of menin, the regulatory mechanism by which menin affects the IGFBP-2 promoter remains unknown.
FUNCTION OF IGFBPS IN METABOLIC SIGNALING
In a transgenic mouse model, overexpression of IGFBP-2 was associated with increased fat accumulation and reduced muscle weight [23]. Although IGFBP-2 is a secondary protein among the circulation binding proteins, the regulatory and functional roles of IGFBP-2 are not well understood compared to those of IGFBP-1. A recent study determined the role of IGFBP-2 to be a pleiotropic oncogenic protein in cancer development [24]. IGFBP-2 stimulates the nuclear form of epidermal growth factor receptor (EGFR), increasing the activated transcription factor 3 (STAT3) pathway. Moreover, overexpression of exogenous IGFBP-2 protein inhibits EGFR activity through suppression of nuclear EGFR signaling [24]. These results demonstrate a strong association between IGFBP-2 and STAT3 and suggest a novel tumor-inducing role for IGFBP2 by providing a linkage between IGFBP-2 and cancer development.
A novel function of IGFBP-2 has been reported in Duchenne muscular dystrophy (DMD) [14]. One study injected IGFBP-2 into the muscles of dystrophic mice and found that muscle-specific upregulation of IGFBP-2 inhibited muscular dystrophy and protected against dystrophic pathophysiology. Although these discoveries could eventually lead to gene therapy for DMD, it is not yet possible to directly address the muscle atrophy resulting from neuromuscular disorders such as DMD. IGF-1 signaling slows progression of DMD [252627], and studies have found that overexpression of IGFBP-2 can assist in dystrophic treatment by reducing muscle composition in a muscle-dystrophic animal model and patients with DMD.
CLINICAL RELEVANCE OF IGFBP-2 LEVELS IN HUMAN SERUM
Chronic hyperinsulinemia reduces IGFBP-2 secretion, resulting in increased availability of IGF-1 [28]. Studies have shown that IGFBPs serve a critical function in the metabolic system and represent an important link between the IGF system and insulin signaling [29]. In recent years, in vivo and in vitro assays, as well as studies in human subjects, have focused on the functional progression of IGFBPs. These investigations have confirmed the role of IGFBPs in metabolic syndrome, including fatty liver, insulin resistance, and obesity [30]. Production of IGFBP-2 is decreased in obese people compared with nonobese individuals, and concentrations of serum IGFBP-2 decrease with increasing body mass index [13]. Several reports have demonstrated that circulating IGFBP-2 concentrations are lower in patients with type 2 diabetes mellitus or obesity [1031]. Because overexpression of IGFBP-2 leads to recovered blood glucose levels in diabetic animal models, and because the antidiabetic drug, metformin, can recover serum IGFBP-2 levels in diabetic patients [10], decreased IGFBP-2 levels may lead to the development of diabetes in humans. Thus, IGFBP-2 may be useful as a biomarker of metabolic disease.
CONCLUSIONS
IGFBP-2 has been shown to be a key regulator of metabolic diseases, such as diabetes and obesity, as well as cancer metabolism. Although IGFBP-2 gene transcription is regulated by several metabolic conditions in endocrine organs, the underlying regulatory mechanism of IGFBP-2 gene expression remains unknown, and further studies are needed. Moreover, the functional roles of IGFBP-2 in metabolic diseases and signaling are still unclear and controversial. Therefore, the relevance of IGFBP-2 to metabolic diseases could be a main driver in the investigation of IGFBP-2's underlying physiological function.
ACKNOWLEDGMENTS
This study was supported by grants from the Korea New Faculty, Korea Research Foundation, Republic of Korea (NRF-2013R1A1A1006606) to S.S.I. and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A1A01057610) to H.S.K.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.