Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(1); 2022 > Article
-
EditorialDiabetes, Obesity and Metabolism Drug Repositioning: Exploring New Indications for Existing Drug-Disease Relationships
-
Hun-Sung Kim1,2

-
Endocrinology and Metabolism 2022;37(1):62-64.
DOI: https://doi.org/10.3803/EnM.2022.1403
Published online: February 28, 2022
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
2Department of Medical Informatics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding author: Hun-Sung Kim. Department of Medical Informatics, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591, Korea, Tel: +82-2-2258-8262, Fax: +82-2-2258-8297, E-mail: 01cadiz@hanmail.net
• Received: January 14, 2022 • Accepted: January 20, 2022
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
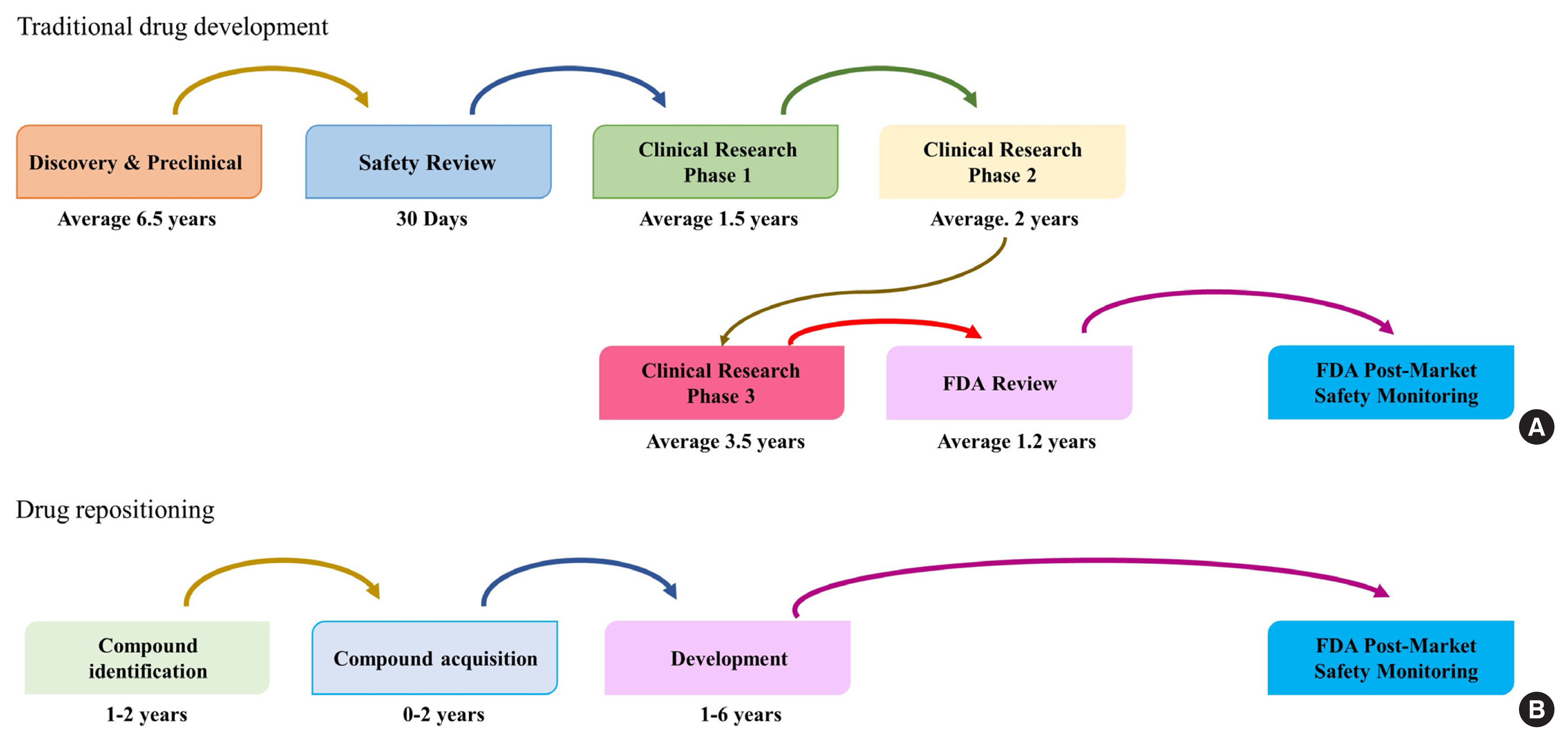
Fig. 1Comparison between (A) traditional drug development and (B) drug repositioning. Modified from Xue et al. [7]. FDA, U.S. Food and Drug Administration.

- 1. Becker RC. Covid-19 treatment update: follow the scientific evidence. J Thromb Thrombolysis 2020;50:43–53.ArticlePubMedPMC
- 2. Jarada TN, Rokne JG, Alhajj R. A review of computational drug repositioning: strategies, approaches, opportunities, challenges, and directions. J Cheminform 2020;12:46.ArticlePubMedPMC
- 3. Yeu Y, Yoon Y, Park S. Protein localization vector propagation: a method for improving the accuracy of drug repositioning. Mol Biosyst 2015;11:2096–102.ArticlePubMed
- 4. Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov 2009;8:959–68.ArticlePubMed
- 5. Saberian N, Peyvandipour A, Donato M, Ansari S, Draghici S. A new computational drug repurposing method using established disease-drug pair knowledge. Bioinformatics 2019;35:3672–8.ArticlePubMedPMC
- 6. Low ZY, Farouk IA, Lal SK. Drug repositioning: new approaches and future prospects for life-debilitating diseases and the COVID-19 pandemic outbreak. Viruses 2020;12:1058.ArticlePubMedPMC
- 7. Xue H, Li J, Xie H, Wang Y. Review of drug repositioning approaches and resources. Int J Biol Sci 2018;14:1232–44.ArticlePubMedPMC
- 8. U.S. Food and Drug Administration. Drug development & approval process [Internet] Silver Spring: FDA; 2019 [cited 2022 Feb 3]. Available from: https://www.fda.gov/drugs/development-approval-process-drugs .
- 9. Nosengo N. Can you teach old drugs new tricks? Nature 2016;534:314–6.ArticlePubMed
- 10. Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 2012;11:191–200.ArticlePubMed
- 11. Nuffer WA, Trujillo JM. Liraglutide: a new option for the treatment of obesity. Pharmacotherapy 2015;35:926–34.ArticlePubMed
- 12. Lotfi Shahreza M, Ghadiri N, Mousavi SR, Varshosaz J, Green JR. A review of network-based approaches to drug repositioning. Brief Bioinform 2018;19:878–92.ArticlePubMed
- 13. Park N, Jeon JY, Jeong E, Kim S, Yoon D. Drug repositioning using temporal trajectories of accompanying comorbidities in diabetes mellitus. Endocrinol Metab (Seoul) 2022;37:65–73.ArticlePubMedPMC
- 14. Kim HS, Kim JH. Proceed with caution when using real world data and real world evidence. J Korean Med Sci 2019;34:e28.ArticlePubMedPMC
- 15. Kim HS, Kim DJ, Yoon KH. Medical big data is not yet available: why we need realism rather than exaggeration. Endocrinol Metab (Seoul) 2019;34:349–54.ArticlePubMedPMC
- 16. Lee S, Kim HS. Prospect of artificial intelligence based on electronic medical record. J Lipid Atheroscler 2021;10:282–90.ArticlePubMedPMC
- 17. Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health 2014;36:e2014008.ArticlePubMedPMC
- 18. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69–77.ArticlePubMedPMC
- 19. Kyoung DS, Kim HS. Understanding and utilizing claim data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) database for research. J Lipid Atheroscler 2021 Nov 26 [Epub]. https://e-jla.org/DOIx.php?id=10.12997/jla.2022.11.e1 .ArticlePDF
- 20. Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci 2018;33:e213.ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Drug Repositioning Using Computer-aided Drug Design (CADD)
Sona Rawat, Kanmani Subramaniam, Selva Kumar Subramanian, Saravanan Subbarayan, Subramanian Dhanabalan, Sashik Kumar Madurai Chidambaram, Balasubramaniam Stalin, Arpita Roy, Nagaraj Nagaprasad, Mahalingam Aruna, Jule Leta Tesfaye, Bayissa Badassa, Ramaswa
Current Pharmaceutical Biotechnology.2024; 25(3): 301. CrossRef - Magic bullets, magic shields, and antimicrobials in between
Praveen Prathapan
Pharmaceutical Science Advances.2023; 1(1): 100002. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite


