Articles
- Page Path
- HOME > Endocrinol Metab > Volume 34(2); 2019 > Article
-
Original ArticleClinical Study Postoperative Thyroid-Stimulating Hormone Levels Did Not Affect Recurrence after Thyroid Lobectomy in Patients with Papillary Thyroid Cancer
-
Myung-Chul Lee1
 , Min Joo Kim2,3
, Min Joo Kim2,3 , Hoon Sung Choi4
, Hoon Sung Choi4 , Sun Wook Cho2
, Sun Wook Cho2 , Guk Haeng Lee1
, Guk Haeng Lee1 , Young Joo Park2
, Young Joo Park2 , Do Joon Park2
, Do Joon Park2
-
Endocrinology and Metabolism 2019;34(2):150-157.
DOI: https://doi.org/10.3803/EnM.2019.34.2.150
Published online: May 10, 2019
1Department of Otorhinolaryngology-Head and Neck Surgery, Korea Cancer Center Hospital, Seoul, Korea.
2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
3Department of Internal Medicine, Healthcare Research Institute, Seoul National University Hospital Healthcare System Gangnam Center, Seoul National University College of Medicine, Seoul, Korea.
4Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea.
- Corresponding author: Min Joo Kim. Department of Internal Medicine, Healthcare Research Institute, Seoul National University Hospital Healthcare System Gangnam Center, 152 Teheran-ro, Gangnam-gu, Seoul 06236, Korea. Tel: +82-2-2112-5513, Fax: +82-2-2112-5794, chorong24@gmail.com
- Corresponding author: Hoon Sung Choi. Department of Internal Medicine, Kangwon National University School of Medicine, 156 Baengnyeong-ro, Chuncheon 24289, Korea. Tel: +82-33-258-9217, Fax: +82-33-258-2404, hoonsung80@gmail.com
Copyright © 2019 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Thyroid-stimulating hormone (TSH) suppression is recommended for patients who undergo thyroidectomy for differentiated thyroid cancer (DTC). However, the impact of TSH suppression on clinical outcomes in low-risk DTC remains uncertain. Therefore, we investigated the effects of postoperative TSH levels on recurrence in patients with low-risk DTC after thyroid lobectomy.
-
Methods
- Patients (n=1,528) who underwent thyroid lobectomy for papillary thyroid carcinoma between 2000 and 2012 were included in this study. According to the mean and dominant TSH values during the entire follow-up period or 5 years, patients were divided into four groups (<0.5, 0.5 to 1.9, 2.0 to 4.4, and ≥4.5 mIU/L). Recurrence-free survival was compared among the groups.
-
Results
- During the 5.6 years of follow-up, 21 patients (1.4%) experienced recurrence. Mean TSH levels were within the recommended low-normal range (0.5 to 1.9 mIU/L) during the total follow-up period or 5 years in 38.1% or 36.0% of patients. The mean and dominant TSH values did not affect recurrence-free survival. Adjustment for other risk factors did not alter the results.
-
Conclusion
- Serum TSH levels did not affect short-term recurrence in patients with low-risk DTC after thyroid lobectomy. TSH suppression should be conducted more selectively.
- The incidence of thyroid cancer has been increasing, and the detection of small thyroid cancers (<1 cm) contributes to a significant portion of the increase [1]. With an increase in the incidence, the need for thyroid lobectomy in patients has also increased. Moreover, as the tumor size criteria for thyroid lobectomy have been widened from 1 to 4 cm [2], thyroid lobectomy is now being performed more often.
- Thyroid-stimulating hormone (TSH) suppression with supraphysiologic doses of levothyroxine is recommended to patients who undergo thyroidectomy for thyroid cancer [2] as TSH suppression can inhibit the proliferation of thyroid cancer cells [34]. Previous studies have reported that TSH suppression improved survival and reduced disease progression and recurrence [5678]. However, effects of TSH suppression were more significant in patients with advanced stage thyroid cancer (stage 3 or 4), and not as significant in patients with early stage thyroid cancer (stage 1 or 2) [56]. In addition, TSH suppression is associated with several morbidities, including cardiovascular and skeletal disease [9] and cardiovascular mortality [10]. TSH suppression therapy is associated with an increased risk of arrhythmias including atrial fibrillation [11] and osteoporosis/bone strength [1121314]. Therefore, TSH suppression should be determined with consideration of these advantages and disadvantages.
- Patients who undergo thyroid lobectomy are typically low-risk patients. Thyroid lobectomy is considered in patients with differentiated thyroid cancer (DTC) <4 cm without extrathyroidal extension (ETE) and clinical evidence of any lymph node (LN) metastases [2]. For these patients, American Thyroid Association (ATA) recommends that TSH may be maintained in the mid to lower reference range (0.5 to 2.0 mU/L) after thyroid lobectomy [2]. However, few studies have supported this recommendation, and whether TSH suppression is required in those patients remains controversial. Therefore, we investigated whether serum TSH levels affect the recurrence in patients who underwent thyroid lobectomy for thyroid cancer.
INTRODUCTION
- Patients
- Patients who underwent thyroid lobectomy for papillary thyroid carcinoma (PTC) at the Korea Cancer Center Hospital and Seoul National University Hospital between January 2000 and December 2012 were included in this study. Patients younger than 20 years of age and subtypes of PTC known to have a poor prognosis such as the tall cell variant were excluded. In total, 1,875 patients met these criteria. We then further excluded patients who were followed less than 2 years (n=166) and who had a measured serum TSH concentration less than 5 during the follow-up period (n=181). Finally, 1,528 patients were included in the analysis (mean age 47 years, female 88%, mean duration of follow-up 5.6 years). Overall, 1,260 patients from the Korea Cancer Center Hospital and 268 from Seoul National University Hospital were included. The study protocol was approved by the Institutional Review Board of Korea Cancer Center Hospital (KIRAMS 2018-04-005) and Seoul National University Hospital (H-1707-035-867). Data were collected retrospectively by the review of electronic medical records.
- Initial operation and follow-up
- Preoperative neck ultrasonography (US) and/or computed tomography was performed in all patients. If there was a nodule (or nodules) found on the contralateral lobe, thyroid lobectomy was performed if the US features of nodules suggested that they were benign or if the results of US-guided fine-needle aspiration (FNA) were benign. Prophylactic central neck dissection was performed depending on the preference of the surgeon.
- After the initial operation, serum TSH concentration and neck US were performed at least once per year. During the 5.6 years of follow-up, the median number of TSH measurements was 9 (range, 5 to 40). When cervical LN or a thyroid mass was suspicious for recurrence on neck US, US-guided FNA or core-needle biopsy was performed. Final diagnosis of the recurrence was confirmed by the pathological or cytological results. The time of recurrence was defined as the date of the first pathological or cytological confirmation.
- Statistical analyses
- Data are expressed as mean±standard deviation. We collected all serum TSH levels measured after surgery. In patients who experienced recurrence only the TSH levels before recurrence were used. After then, mean TSH values were calculated (TSHtotal). Serum TSH levels at each follow-up visit were categorized into four groups (<0.5, 0.5 to 1.9, 2.0 to 4.4, and ≥4.5 mIU/L). The dominant TSH value in each patient was defined as the TSH group comprising most proportion among sequential TSH groups during follow-up period. In the analysis with dominant TSH value, 164 patients who showed multiple peaks of serum TSH levels were excluded because we could not determine the dominant TSH value. In addition, mean and dominant TSH5yrs values were calculated with serum TSH levels for 5 years after surgery. Categorical and continuous variables were analyzed using the chi-square test and Student's t test, respectively. Recurrence-free survival (RFS) was defined as the time interval between the date of thyroid surgery and the date of recurrence. RFS curves were plotted using the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazard regression was also performed. In multivariate Cox regression analysis, known risk factors such as age, sex, tumor size, multiplicity, ETE, and LN metastasis were included for adjustment. P<0.05 was considered significant. All statistical analyses were performed by SPSS version 23.0 for Windows (IBM Co., Armonk, NY, USA).
METHODS
- Baseline characteristics
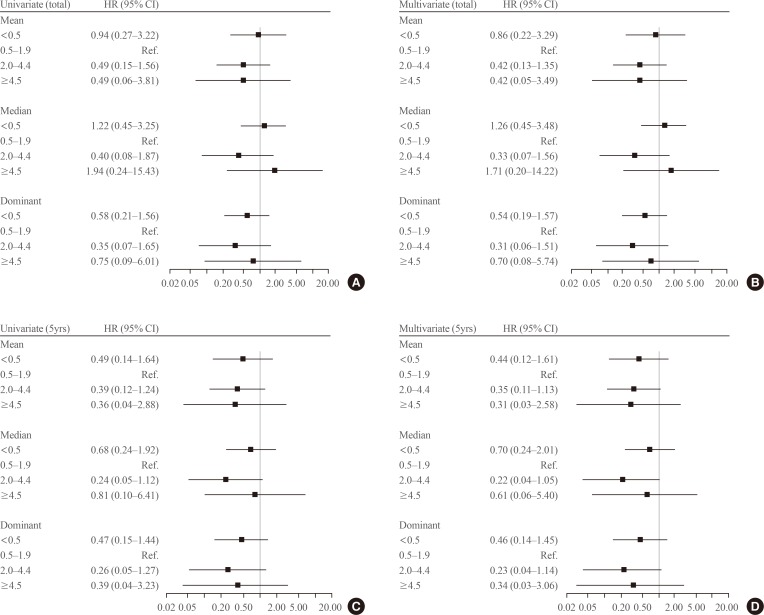
- Baseline characteristics of patients are summarized in Table 1. Most patients (89%) had conventional PTC and 87% of the tumors were less than 1 cm. However, 34% and 13% had ETE and central LN metastasis, respectively. During the 5.6 years of follow-up, 21 patients (1.4%) experienced recurrence, and were locoregional; there was no distant metastasis. Among these patients, 14 patients experienced recurrence due to contralateral thyroid nodules and seven patients did due to LN metastasis. All patients underwent completion thyroidectomy and LN dissection. On the final surgical pathology, 16 patients had PTC in the contralateral lobe, and 10 had LN metastasis. The median time to recurrence was 4.9 years (range, 2.1 to 17.0). The 5 and 10-year recurrence rates were 0.8% and 1.4%, respectively. Among the known risk factors for recurrence, only tumor size had a significant impact on the RFS, but the others did not (Fig. 1A, Supplemental Table S1).
- Distribution of postoperative TSH levels
- According to the mean and dominant TSHtotal or TSH5yrs, patients were divided into four groups (Table 2). In 36.0% of patients, mean TSH5yrs levels were within the recommended low-normal range (0.5 to 1.9 mIU/L). The mean TSH5yrs level in 46.7% of patients were in the high-normal range (2.0 to 4.4 mIU/L). Overall, 7.5% or 9.8% of patients had a mean TSH5yrs level of <0.5 or ≥4.5 mIU/L, respectively. The distribution of dominant TSH values were similar (Table 2), and patients with dominant TSH5yrs values within the recommended low-normal range (0.5 to 1.9 mIU/L) were 31.0%.
- Impact of serum TSH levels on tumor recurrence
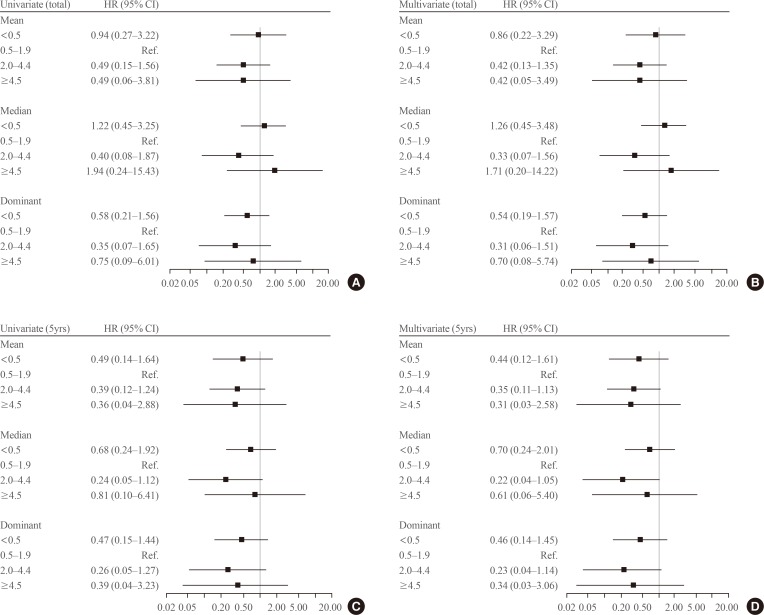
- We evaluated the effects of serum TSH levels on RFS. Kaplan-Meier RFS curves showed that mean and dominant TSH values did not affect RFS (Fig. 1B, C). Both univariate and multivariate Cox regression analyses with several known risk factors including age, sex, tumor size, multiplicity, ETE, and LN metastasis showed no significant differences in the results (Fig. 2).
- There is a possibility that serum TSH levels were suppressed differently based on the characteristics of the patients. Therefore, we compared the baseline characteristics among the groups according to the mean TSH5yrs values (Table 1). Compared to the mean TSH5yrs 0.5 to 1.9 group, the mean TSH5yrs <0.5 group showed a larger tumor size (0.9±0.7 cm vs. 0.7±0.5 cm), and the mean TSH5yrs 2.0 to 4.4 group experienced fewer instances of LN metastasis (11% vs. 17%). Analysis with the dominant TSH values yielded similar results (data not shown). Therefore, we conducted a subgroup analysis according to tumor size and LN metastasis. However, mean TSH5yrs values did not affect RFS in the subgroup analysis (Supplemental Table S2), and dominant TSH values were the same (data not shown).
- Impact of levothyroxine use on tumor recurrence
- Among all patients, 1,087 (71%) had taken levothyroxine. The mean TSH5yrs levels of these patients was significantly lower than that in patients without levothyroxine (2.46 mIU/L vs. 2.97 mIU/L, P<0.01). The lower TSH5yrs group, levothyroxine was more significantly prescribed (Table 1). Levothyroxine use did not affect RFS (hazard ratio, 1.74; 95% confidence interval, 0.50 to 5.99; P=0.38) (Fig. 1D).
RESULTS
- In the present study, we demonstrated that postoperative serum TSH levels did not affect recurrence in low-risk PTC patients who underwent thyroid lobectomy. These findings suggest that 20.00there is no evidence on the beneficial effect of TSH suppression on the short-term recurrence.
- There is controversy regarding TSH suppression in low-risk DTC patients who undergo thyroid lobectomy because of a lack of evidence. Previous studies have shown that TSH suppression was associated with a decrease in disease recurrence or death [7]; however, those studies included patients at various tumor stages and risks. Therefore, it is difficult to apply the results equally to low-risk DTC patients. The National Thyroid Cancer Treatment Cooperative Study Group (NTCTCS), a large cohort of 11 North American institution, has reported the association between TSH suppression and clinical outcomes [5615]. First, Cooper et al. [5] reported that TSH suppression did not predict disease progression in patients with stage I or II DTC. Next, Jonklaas et al. [6] reported that TSH suppression did not affect disease-specific survival in patients with stage I or II DTC. However, in the most recent study, Carhill et al. [15] reported that modest TSH suppression was associated with improved DFS and overall survival in all stages. However, in a randomized controlled trial in which most subjects (88%) had low-risk, TSH suppression targeted at TSH <0.01 µU/mL did not alter RFS [16]. Among patients with stage I or II DTC, those who undergo thyroid lobectomy typically have a lower risk. Recently, a study with low-risk DTC patients who underwent thyroid lobectomy was reported for the first time, and RFS did not differ according to TSH suppressive therapy or serum TSH levels [17]. To verify the impact of serum TSH levels in low-risk DTC patients who underwent thyroid lobectomy, we conducted the present study with more patients, and the same results were confirmed. TSH suppression in these patients is not necessary and should be reconsidered.
- Furthermore, TSH suppression has adverse effects. It can cause arrhythmias, including atrial fibrillation [11] and osteoporosis [1121314], and increase cardiovascular mortality [10]. Considering these adverse effects, TSH suppression should not be performed without a clear evidence of its effectiveness in clinical outcomes.
- Thyroid lobectomy may not require life-long thyroid hormone replacement because the contralateral thyroid is left intact. It is one of the major reasons for selecting thyroid lobectomy. However, a recent American study found that 73% of patients at 1 year after thyroid lobectomy had TSH >2 mU/L [18]. This suggests that approximately 73% of patients after thyroid lobectomy will need levothyroxine only for TSH suppression according to the current ATA guideline [2]. If TSH suppression is not necessary in low-risk DTC patients who undergo thyroid lobectomy, many patients may no longer need daily medication. In reality, 29% showed persistent hypothyroidism and only 12% to 13% of patients required levothyroxine replacement after thyroid lobectomy without TSH suppression [1920].
- This study has some limitations considering its retrospective design. The recurrence rate was very low because of the nature of low-risk PTC, and previous studies reported that the 10-year recurrence rate was 1.7% to 8.2% [212223]. In this study, the recurrence rate (1.4%) was slightly lower than the previous ones, which can weaken the power of results. Moreover, we cannot determine the effects of serum TSH levels on long-term recurrence because of a short follow-up duration (5.6 years). Thyroid cancers recur steadily even after 5 years [2425]. Therefore, although a significant impact of postoperative TSH levels on the recurrence was not found in this study, TSH suppression can be effective in the long-term clinical outcomes and further studies are warranted in this regard. Lastly, the TSH evaluation period differed individually. TSH5yrs values are estimated using serum TSH levels for up to 5 years after surgery. In our study, the median time to recurrence was 4.9 years, that means about half of the patients had recurrence before 5 years. Therefore, the TSH evaluation period was shorter in patients with recurrence at less than 5 years, compared to the patients without recurrence. This limitation would be avoided by using serum TSH levels until earlier time after surgery, but it was impossible because of the insufficient number of TSH measurements. When using serum TSH levels for up to 2 years after surgery, the proportion of patients who measured serum TSH levels less than five times was 65%.
- In the present study, serum TSH levels did not affect the short-term recurrence in patients with low-risk DTC after thyroid lobectomy. Overall, TSH suppression is more useful in terms of patient-specific considerations and is not be necessarily useful for all patients. Therefore, the decision for TSH suppression should be made carefully, particularly in older patients with a short life expectancy or many risk factors for TSH suppression-related adverse events, including arrhythmia or osteoporosis/fractures.
DISCUSSION
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS:
Article information
SUPPLEMENTARY MATERIALS
Supplemental Table S1
Supplemental Table S2
- 1. Moon JH, Kim KM, Oh TJ, Choi SH, Lim S, Park YJ, et al. The effect of TSH suppression on vertebral trabecular bone scores in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 2017;102:78–85. ArticlePubMed
- 2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1–133. ArticlePubMedPMC
- 3. Pötter E, Horn R, Scheumann GF, Dralle H, Costagliola S, Ludgate M, et al. Western blot analysis of thyrotropin receptor expression in human thyroid tumours and correlation with TSH-binding. Biochem Biophys Res Commun 1994;205:361–367. ArticlePubMed
- 4. Brabant G. Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab 2008;93:1167–1169. ArticlePubMedPDF
- 5. Cooper DS, Specker B, Ho M, Sperling M, Ladenson PW, Ross DS, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998;8:737–744. ArticlePubMed
- 6. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229–1242. ArticlePubMed
- 7. McGriff NJ, Csako G, Gourgiotis L, Lori CG, Pucino F, Sarlis NJ. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002;34:554–564. ArticlePubMed
- 8. Hovens GC, Stokkel MP, Kievit J, Corssmit EP, Pereira AM, Romijn JA, et al. Associations of serum thyrotropin concentrations with recurrence and death in differentiated thyroid cancer. J Clin Endocrinol Metab 2007;92:2610–2615. ArticlePubMedPDF
- 9. Parker WA, Edafe O, Balasubramanian SP. Long-term treatment-related morbidity in differentiated thyroid cancer: a systematic review of the literature. Pragmat Obs Res 2017;8:57–67. ArticlePubMedPMC
- 10. Klein Hesselink EN, Klein Hesselink MS, de Bock GH, Gansevoort RT, Bakker SJ, Vredeveld EJ, et al. Long-term cardiovascular mortality in patients with differentiated thyroid carcinoma: an observational study. J Clin Oncol 2013;31:4046–4053. ArticlePubMed
- 11. Pajamaki N, Metso S, Hakala T, Ebeling T, Huhtala H, Ryodi E, et al. Long-term cardiovascular morbidity and mortality in patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2018;88:303–310. ArticlePubMed
- 12. Wang LY, Smith AW, Palmer FL, Tuttle RM, Mahrous A, Nixon IJ, et al. Thyrotropin suppression increases the risk of osteoporosis without decreasing recurrence in ATA low- and intermediate-risk patients with differentiated thyroid carcinoma. Thyroid 2015;25:300–307. ArticlePubMedPMC
- 13. Tournis S, Antoniou JD, Liakou CG, Christodoulou J, Papakitsou E, Galanos A, et al. Volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in women with differentiated thyroid cancer under TSH suppression. Clin Endocrinol (Oxf) 2015;82:197–204. ArticlePubMed
- 14. Sugitani I, Fujimoto Y. Effect of postoperative thyrotropin suppressive therapy on bone mineral density in patients with papillary thyroid carcinoma: a prospective controlled study. Surgery 2011;150:1250–1257. ArticlePubMed
- 15. Carhill AA, Litofsky DR, Ross DS, Jonklaas J, Cooper DS, Brierley JD, et al. Long-term outcomes following therapy in differentiated thyroid carcinoma: NTCTCS registry analysis 1987–2012. J Clin Endocrinol Metab 2015;100:3270–3279. ArticlePubMedPMC
- 16. Sugitani I, Fujimoto Y. Does postoperative thyrotropin suppression therapy truly decrease recurrence in papillary thyroid carcinoma? A randomized controlled trial. J Clin Endocrinol Metab 2010;95:4576–4583. ArticlePubMed
- 17. Park S, Kim WG, Han M, Jeon MJ, Kwon H, Kim M, et al. Thyrotropin suppressive therapy for low-risk small thyroid cancer: a propensity score-matched cohort study. Thyroid 2017;27:1164–1170. ArticlePubMed
- 18. Cox C, Bosley M, Southerland LB, Ahmadi S, Perkins J, Roman S, et al. Lobectomy for treatment of differentiated thyroid cancer: can patients avoid postoperative thyroid hormone supplementation and be compliant with the American Thyroid Association guidelines? Surgery 2018;163:75–80. ArticlePubMed
- 19. Park S, Jeon MJ, Song E, Oh HS, Kim M, Kwon H, et al. Clinical features of early and late postoperative hypothyroidism after lobectomy. J Clin Endocrinol Metab 2017;102:1317–1324. ArticlePubMed
- 20. Ebina A, Sugitani I, Fujimoto Y, Yamada K. Risk-adapted management of papillary thyroid carcinoma according to our own risk group classification system: is thyroid lobectomy the treatment of choice for low-risk patients? Surgery 2014;156:1579–1588. ArticlePubMed
- 21. Park YM, Lee DY, Oh KH, Cho JG, Baek SK, Kwon SY, et al. Clinical implications of pathologic factors after thyroid lobectomy in patients with papillary thyroid carcinoma. Oral Oncol 2017;75:1–5. ArticlePubMed
- 22. Kim SK, Park I, Woo JW, Lee JH, Choe JH, Kim JH, et al. Total thyroidectomy versus lobectomy in conventional papillary thyroid microcarcinoma: analysis of 8,676 patients at a single institution. Surgery 2017;161:485–492. ArticlePubMed
- 23. Matsuzu K, Sugino K, Masudo K, Nagahama M, Kitagawa W, Shibuya H, et al. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg 2014;38:68–79. ArticlePubMedPDF
- 24. Hwangbo Y, Kim JM, Park YJ, Lee EK, Lee YJ, Park DJ, et al. Long-term recurrence of small papillary thyroid cancer and its risk factors in a Korean multicenter study. J Clin Endocrinol Metab 2017;102:625–633. ArticlePubMedPDF
- 25. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994;97:418–428. ArticlePubMed
References
Kaplan-Meier curves of recurrence-free survival (RFS). (A) Tumor size. (B) Mean thyroid-stimulating hormone levels for 5 years after surgery (TSH5yrs) values. (C) Dominant TSH5yrs values. (D) Levothyroxine use.

Forest plot for recurrence-free survival according to the mean thyroid-stimulating hormone (TSH) values during the total follow-up period or 5 years. In multivariate Cox regression analysis, known risk factors including age, sex, tumor size, multiplicity, extrathyroidal extension, and lymph node metastasis were adjusted. (A) Univariate Cox regression analysis with mean TSH values were calculated (TSHtotal) values. (B) Multivariate Cox regression analysis with mean TSHtotal values. (C) Univariate Cox regression analysis with mean TSH levels for 5 years after surgery (TSH5yrs) values. (D) Multivariate Cox regression analysis with mean TSH5yrs values. HR, hazard ratio; CI, confidence interval.
Baseline Characteristics of Patients According to the Mean TSH5yrs Value
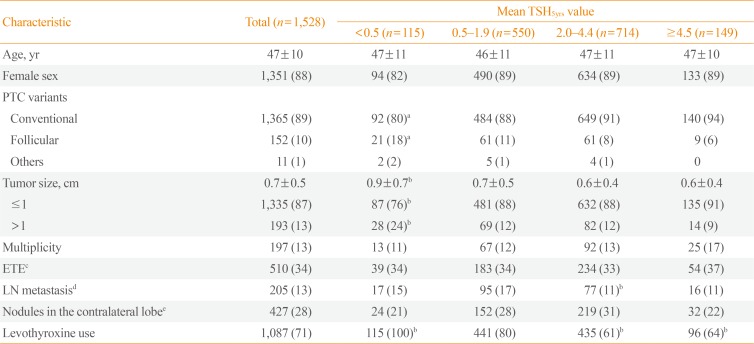
Values are expressed as mean±SD or number (%).
TSH5yrs, mean thyroid-stimulating hormone levels for 5 years after surgery; PTC, papillary thyroid carcinoma; ETE, extrathyroidal extension; LN, lymph node.
aP<0.05, bP<0.01 compared mean TSH5yrs 0.5–1.9 group; cTo check ETE was unavailable in 14 PTCs; dAll was central compartment LN metastasis; ePreoperative images were unavailable in 12 patients.
Distribution of TSH Suppression
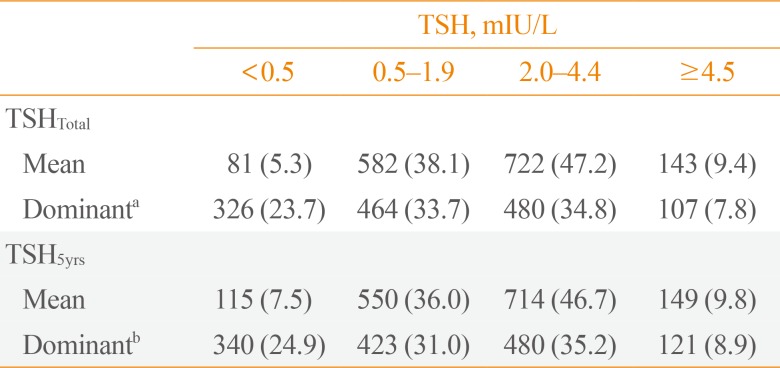
Values are expressed as number (%).
TSH, thyroid-stimulating hormone; TSHTotal, TSH values were calculated; TSH5yrs, TSH levels for 5 years after surgery.
a151 patients who were difficult to classify were excluded from this analysis; b164 patients who were difficult to classify were excluded from this analysis.
Figure & Data
References
Citations

- Dynamic risk assessment in patients with differentiated thyroid cancer
Erika Abelleira, Fernando Jerkovich
Reviews in Endocrine and Metabolic Disorders.2024; 25(1): 79. CrossRef - Stimulating thyroglobulin to TSH ratio predict long-term efficacy of 131I therapy in patients with differentiated thyroid cancer after total thyroidectomy: a retrospective study
Xue Yin, Chao Lu, Danyang Sun, Yanhui Ji, Yan Wang, Hongyuan Zheng, Ziyu Ma, Qiang Jia, Jian Tan, Wei Zheng
Endocrine.2024;[Epub] CrossRef - Effect of thyroid-stimulating hormone suppression on quality of life in thyroid lobectomy patients: interim analysis of a multicenter, randomized controlled trial in low- to intermediate-risk thyroid cancer patients (MASTER study)
Ja Kyung Lee, Eu Jeong Ku, Su-jin Kim, Woochul Kim, Jae Won Cho, Kyong Yeun Jung, Hyeong Won Yu, Yea Eun Kang, Mijin Kim, Hee Kyung Kim, Junsun Ryu, June Young Choi
Annals of Surgical Treatment and Research.2024; 106(1): 19. CrossRef - Impact of a mobile health intervention based on multi-theory model of health behavior change on self-management in patients with differentiated thyroid cancer: protocol for a randomized controlled trial
Yang Jiang, Xiangju Sun, Maomin Jiang, Hewei Min, Jing Wang, Xinghua Fu, Jiale Qi, Zhenjie Yu, Xiaomei Zhu, Yibo Wu
Frontiers in Public Health.2024;[Epub] CrossRef - Outcomes and Trends of Treatments in High‐Risk Differentiated Thyroid Cancer
Arash Abiri, Khodayar Goshtasbi, Sina J. Torabi, Edward C. Kuan, William B. Armstrong, Tjoson Tjoa, Yarah M. Haidar
Otolaryngology–Head and Neck Surgery.2023; 168(4): 745. CrossRef - Outcomes of Patients with an Intermediate‐Risk Group According to the Japanese Risk Classification of Papillary Thyroid Carcinoma
Kiyomi Horiuchi, Mikiko Fujimoto, Kamio Hidenori, Yusaku Yoshida, Eiichiro Noguchi, Yoko Omi, Takahiro Okamoto
World Journal of Surgery.2023; 47(10): 2464. CrossRef - Effects of Isthmus Preservation on Postoperative Hypothyroidism after Lobectomy
Yeong San Jeon, Wan Wook Kim
International Journal of Thyroidology.2023; 16(1): 120. CrossRef - Physical activity and reduced risk of fracture in thyroid cancer patients after thyroidectomy — a nationwide cohort study
Jinyoung Kim, Kyungdo Han, Jin-Hyung Jung, Jeonghoon Ha, Chaiho Jeong, Jun-Young Heu, Se-Won Lee, Jeongmin Lee, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Ki-Hyun Baek
Frontiers in Endocrinology.2023;[Epub] CrossRef - Association between thyroid stimulating hormone levels and papillary thyroid cancer risk: A meta-analysis
Bin Xu, Shu-Yan Gu, Ning-Ming Zhou, Jun-Jie Jiang
Open Life Sciences.2023;[Epub] CrossRef - The Relationship between Thyrotropin Serum Concentrations and Thyroid Carcinoma
Xueqi Zhang, Lijun Tian, Di Teng, Weiping Teng
Cancers.2023; 15(20): 5017. CrossRef - Differentiated thyroid cancer: a focus on post-operative thyroid hormone replacement and thyrotropin suppression therapy
Benjamin J. Gigliotti, Sina Jasim
Endocrine.2023; 83(2): 251. CrossRef - Optimal Serum Thyrotropin Level for Patients with Papillary Thyroid Carcinoma After Lobectomy
Siyuan Xu, Ying Huang, Hui Huang, Xiaohang Zhang, Jiaxin Qian, Xiaolei Wang, Zhengang Xu, Shaoyan Liu, Jie Liu
Thyroid.2022; 32(2): 138. CrossRef - Optimal Thyrotropin Following Lobectomy for Papillary Thyroid Cancer: Does It Exist?
Lindsay Bischoff, Megan R. Haymart
Thyroid.2022; 32(2): 117. CrossRef - Evaluation of ITGA3 as a Biomarker of Progression and Recurrence in Papillary Thyroid Carcinoma
Guoliang Zhang, Bing Li, Yuanmei Lin
Frontiers in Oncology.2022;[Epub] CrossRef - The Question of an Optimal TSH Goal After Lobectomy for Papillary Thyroid Cancer
Bernadette Biondi
Clinical Thyroidology.2022; 34(2): 67. CrossRef - Is Maintaining Thyroid-Stimulating Hormone Effective in Patients Undergoing Thyroid Lobectomy for Low-Risk Differentiated Thyroid Cancer? A Systematic Review and Meta-Analysis
Ho-Ryun Won, Eonju Jeon, Jae Won Chang, Yea Eun Kang, Kunho Song, Sun Wook Kim, Dong Mee Lim, Tae Kwun Ha, Ki-Wook Chung, Hyo-Jeong Kim, Young Joo Park, Bon Seok Koo
Cancers.2022; 14(6): 1470. CrossRef - Research Review of Thermal Ablation in the Treatment of Papillary Thyroid Carcinoma
Di Ou, Chen Chen, Tian Jiang, Dong Xu
Frontiers in Oncology.2022;[Epub] CrossRef - CACA guidelines for holistic integrative management of thyroid cancer
Minghua Ge, Ming Gao, Ruochuan Cheng, Xiaohong Chen, Haixia Guan, Yansong Lin, Shaoyan Liu, Yu Wang, Chuanming Zheng, Xiangqian Zheng
Holistic Integrative Oncology.2022;[Epub] CrossRef - Value of thyroglobulin post hemithyroidectomy for cancer: a literature review
Saam S. Tourani, Bill Fleming, Justin Gundara
ANZ Journal of Surgery.2021; 91(4): 724. CrossRef - Pros and cons of hemi‐thyroidectomy for low‐risk differentiated thyroid cancer
Alexander J. Papachristos, Anthony Glover, Mark S. Sywak, Stan B. Sidhu
ANZ Journal of Surgery.2021; 91(9): 1704. CrossRef - Management and follow-up of differentiated thyroid cancer not submitted to radioiodine treatment: a systematic review
Carla GAMBALE, Rossella ELISEI, Antonio MATRONE
Minerva Endocrinologica.2021;[Epub] CrossRef - The Recovery of Thyroid Function in Low-Risk Papillary Thyroid Cancer After Lobectomy: A 3-Year Follow-Up Study
Yi Dou, Yingji Chen, Daixing Hu, Xinliang Su
Frontiers in Endocrinology.2021;[Epub] CrossRef - Controversy: For or against thyroid lobectomy in > 1 cm differentiated thyroid cancer?
Fabrice Menegaux, Jean-Christophe Lifante
Annales d'Endocrinologie.2021; 82(2): 78. CrossRef - Thyroid cancer, recent advances in diagnosis and therapy
Fadi Nabhan, Priya H. Dedhia, Matthew D. Ringel
International Journal of Cancer.2021; 149(5): 984. CrossRef - A Multicenter, Randomized, Controlled Trial for Assessing the Usefulness of Suppressing Thyroid Stimulating Hormone Target Levels after Thyroid Lobectomy in Low to Intermediate Risk Thyroid Cancer Patients (MASTER): A Study Protocol
Eun Kyung Lee, Yea Eun Kang, Young Joo Park, Bon Seok Koo, Ki-Wook Chung, Eu Jeong Ku, Ho-Ryun Won, Won Sang Yoo, Eonju Jeon, Se Hyun Paek, Yong Sang Lee, Dong Mee Lim, Yong Joon Suh, Ha Kyoung Park, Hyo-Jeong Kim, Bo Hyun Kim, Mijin Kim, Sun Wook Kim, Ka
Endocrinology and Metabolism.2021; 36(3): 574. CrossRef - Long-term follow-up results of PTMC treated by ultrasound-guided radiofrequency ablation: a retrospective study
Yalin Zhu, Ying Che, Shuhang Gao, Shuangsong Ren, Mengying Tong, Lina Wang, Fang Yang
International Journal of Hyperthermia.2021; 38(1): 1225. CrossRef - Thyroid Hormone Supplementation Therapy for Differentiated Thyroid Cancer After Lobectomy: 5 Years of Follow-Up
Soo Young Kim, Hee Jun Kim, Seok-Mo Kim, Hojin Chang, Yong Sang Lee, Hang-Seok Chang, Cheong Soo Park
Frontiers in Endocrinology.2020;[Epub] CrossRef - Thyroid Lobectomy for Low to Intermediate Risk Differentiated Thyroid Cancer
Dana M. Hartl, Joanne Guerlain, Ingrid Breuskin, Julien Hadoux, Eric Baudin, Abir Al Ghuzlan, Marie Terroir-Cassou-Mounat, Livia Lamartina, Sophie Leboulleux
Cancers.2020; 12(11): 3282. CrossRef - Annual Neck Ultrasonography Surveillance between 3 to 12 Years after Thyroid Lobectomy for Papillary Thyroid Microcarcinoma
Jin Gu Kang, Jung Eun Choi, Soo Jung Lee, Su Hwan Kang
International Journal of Thyroidology.2020; 13(2): 142. CrossRef - Thyroid hormone therapy in differentiated thyroid cancer
Giorgio Grani, Valeria Ramundo, Antonella Verrienti, Marialuisa Sponziello, Cosimo Durante
Endocrine.2019; 66(1): 43. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite