Articles
- Page Path
- HOME > Endocrinol Metab > Volume 32(1); 2017 > Article
-
Review ArticleThe SCAP/SREBP Pathway: A Mediator of Hepatic Steatosis
-
Young-Ah Moon

-
Endocrinology and Metabolism 2017;32(1):6-10.
DOI: https://doi.org/10.3803/EnM.2017.32.1.6
Published online: January 19, 2017
Department of Molecular Medicine, Inha University School of Medicine, Incheon, Korea.
- Corresponding author: Young-Ah Moon. Department of Molecular Medicine, Inha University School of Medicine, 100 Inha-ro, Nam-gu, Incheon 22212, Korea. Tel: +82-32-860-9833, Fax: +82-32-885-8302, yamoon15@inha.ac.kr
• Received: October 26, 2016 • Revised: November 11, 2016 • Accepted: November 20, 2016
Copyright © 2017 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Nonalcoholic fatty liver disease (NAFLD) is strongly associated with insulin resistance, obesity, and dyslipidemia. NAFLD encompasses a wide range of states from the simple accumulation of triglycerides in the hepatocytes to serious states accompanied by inflammation and fibrosis in the liver. De novo lipogenesis has been shown to be a significant factor in the development of hepatic steatosis in insulin-resistant states. Sterol regulatory element binding protein-1c (SREBP-1c) is the main transcription factor that mediates the activation of lipogenesis, and SREBP cleavage activating protein (SCAP) is required for the activation of SREBPs. Here, recent animal studies that suggest SCAP as a therapeutic target for hepatic steatosis and hypertriglyceridemia are discussed.
- Nonalcoholic fatty liver disease (NAFLD) is a medical condition characterized by the accumulation of fat in the liver. NAFLD is used to describe a range of states from simple accumulation of triglycerides (TG) in hepatocytes to the condition with inflammation, fibrosis, and cirrhosis. The prevalence of NAFLD has been reported in the range of 10% to 24% of normal and 58% to 74% of obese people, depending on the population studied and the analytic methods used [12]. The simple accumulation of TG in hepatocytes is not considered a serious disease condition and is often self-limited; however, 4% to 27% of the patients with severe fatty liver that is not treated properly could develop cirrhosis, which can ultimately develop into hepatocellular carcinoma [3].
- Various drugs, hormones, and multiple genetic defects in energy metabolism can cause fat accumulation in the liver. However, the most common cause of NAFLD is insulin resistance, which is closely related with obesity, diabetes, and dyslipidemia [45]. Changing lifestyles including weight loss, diet, and exercise to control the related conditions such as obesity and diabetes is the treatment of choice for fatty liver diseases. Drugs that improve insulin resistance and dyslipidemia, antioxidants, and bile acids are currently used for treatments, but the effects are limited, and some of the drugs often produce serious side effects. This review will discuss molecular changes that mediate hepatic TG accumulation in insulin resistance and focus on recent animal studies that suggest a new therapeutic target for hepatic steatosis and hypertriglyceridemia.
INTRODUCTION
- The fatty acids for hepatic TG are derived from three sources: (1) dietary fat, (2) free fatty acids released from adipose tissue, and (3) hepatic de novo lipogenesis. A series of metabolic alterations occur in the liver in insulin-resistant states. In normal people, 80% of hepatic fatty acids are derived from adipose tissue, while those from de novo lipogenesis and diet account for 5% and 15%, respectively. However, in obese and hyperinsulinemic NAFLD patients, the portion from de novo lipogenesis is increased to 26% [6]. In livers of rodent models of insulin resistance, increased rates of fatty acid synthesis are a significant contributor of the development of hepatic steatosis [7]. In the setting of increased lipogenesis, mitochondrial fatty acid oxidation is reduced. In adipocytes, hormone-sensitive lipase activity is increased in insulin resistance, which results in continuous release of free fatty acid through TG lipolysis. Therefore, in an insulin-resistant state, the increased influx of fatty acids from adipocytes, increased de novo lipogenesis, and reduced fatty acid oxidation result in TG accumulation in the liver (Fig. 1).
SOURCES OF HEPATIC TRIGLYCERIDES
- Hepatic de novo lipogenesis is mainly regulated by transcriptional control of the genes involved in the process by transcription factors such as sterol regulatory element binding protein-1c (SREBP-1c), carbohydrate response element binding protein (ChREBP), liver X receptor α, and peroxisome proliferator-activated receptor γ [8910]. Among these, SREBP-1c is the main transcription factor that regulates hepatic de novo lipogenesis by insulin.
- SREBP-1c is one of three SREBP isoforms (SREBP-1a, SREBP-1c, and SREBP-2) that belong to the basic helix-loop-helix-leucine zipper family of transcription factors. SREBP-1a and 1c are derived from a single gene using different promoters and exon 1s. The longer acidic NH2-terminus of SREBP-1a makes it more potent than SREBP-1c and can activate all SREBP-responsive genes that mediate the synthesis of fatty acids, TG, and cholesterol. The roles of SREBP-1c and SREPB-2 are more restricted. SREBP-1c preferentially enhances transcription of genes required for fatty acid synthesis, while SREBP-2 preferentially activates genes required for cholesterol synthesis. SREBP-1c and SREBP-2 are predominant isoforms in the liver and most other intact tissues, and SREBP-1c is regulated by insulin [8].
- SREBPs are synthesized as inactive precursors that are bound to the endoplasmic reticulum (ER) membrane through two hydrophobic transmembrane-spanning segments connected by a short loop. The NH2-terminal domain of SREBP is the transcription factor region that must be released proteolytically by the two proteases on Golgi apparatus. SREBP cleavage activating protein (SCAP) is required in this process by escorting SREBP from the ER to the Golgi as well as by sensing sterol level in the cell. Other players in the SREBP process are INSIGs (insulin induced genes), which are associated with SCAP. Dissociation of INSIGs from the SCAP-SREBP complex is required for the SREBP-SCAP complex to move to the Golgi [1112]. The released NH2-terminal domain enters the nucleus where it activates multiple target genes by binding to the SREs (sterol response elements) in their promoter (Fig. 2). Enzymes that catalyze the synthesis of fatty acids, TG, and NADPH required for fatty acid synthesis are regulated by SREBP-1c. The typical genes regulated by SREBP-1c are ATP-citrate lyase, acetyl-coenzyme A (CoA) carboxylase, fatty acid synthase, ELOVL6 (elongation of long chain fatty acids family member 6), stearoyl-CoA desaturase, glycerol-3-phosphate acyl transferase, malic enzyme, and glucose 6-phosphate dehydrogenase [8]. The in vivo role of SREBP-1c was demonstrated in a transgenic mouse model that overexpresses SREBP-1c in the liver, which leads to the development of hepatic steatosis due to the increase in lipogenesis [13].
- Increased rates of hepatic fatty acid synthesis contribute to the development of hepatic steatosis in rodent models of insulin-resistance and obesity [7]. The leptin-deficient ob/ob mouse is a model of insulin resistance and severe obesity that is commonly used in metabolic studies. Hyperglycemia, hyperinsulinemia, and hyperphagia along with insulin resistance and severe obesity are the representative characteristics of this model. Hyperinsulinemia in ob/ob mice leads to hepatic SREBP-1c activation and hepatic steatosis, while hepatic glucose production is increased due to insulin resistance. The deletion of the SREBP-1c gene in the liver of ob/ob mice results in an approximately 50% reduction of hepatic TG, which indicates a significant role of SREBP-1c in the hepatic steatosis exhibited in the ob/ob mouse, a model of insulin resistance [14]. Compensatory activation of SREBP-2 in the absence of SREBP-1c and the activation of ChREBP by high glucose level are expected for the residual elevation of TG in the liver of this model [15]. ChREBP is a transcription factor that is independently activated by glucose rather than insulin. It activates liver pyruvate kinase, which generates pyruvate, a source of acetyl-CoA, from phosphoenolpyruvate, as well as genes involved in fatty acid synthesis [16].
REGULATION OF DE NOVO LIPOGENESIS BY SREBP TRANSCRIPTION FACTORS
- SCAP is required for the activation of all three isoforms of SREBP. Disruption of Scap in the liver precludes proteolytic cleavage of the NH2-terminal region of SREBPs and abolishes the nuclear forms of all SREBP-1 and -2 in the liver. When Scap is deleted in the liver of normal mouse lines (L-Scap-/-), the expression of target genes in both the cholesterol and the fatty acid synthetic pathways are reduced, and the rates of fatty acid and cholesterol synthesis are diminished by 70% to 80% in the liver [17]. In ob/ob mice, the mRNAs of the genes involved in fatty acid synthesis are markedly elevated because of the SREBP-1c activation. The Scap deletion in the liver of ob/ob mice (L-Scap-/-; ob/ob) abolishes this elevation of lipogenic gene expression, which results in a 90% reduction in fatty acid synthesis rate. The large pale livers in ob/ob mice caused by the massive engorgement with TG are restored to a normal shape. In fact, the hepatic TG contents are completely resolved to normal or even lower than normal levels. The same effects are observed in the insulin resistance model induced by long-term feeding of a high-fat diet. Despite the abolishment of hepatic steatosis in L-Scap-/-; ob/ob mice, the plasma insulin and plasma glucose levels remain high. They fail to improve intolerance to glucose or insulin challenges shown in ob/ob mice with hepatic steatosis. L-Scap-/-; ob/ob mice are one example showing that hepatic fatty acid overproduction is not required for the development of systemic insulin resistance in mice, and hepatic steatosis and insulin resistance may be independent conditions [14].
- In humans, dyslipidemia is commonly associated with hepatic steatosis and insulin resistance. Evidence has shown that NAFLD is an independent risk factor of atherogenic dyslipidemia in various clinical studies [1819]. Hamsters easily develop insulin resistance and dyslipidemia after several weeks of being fed a high-fructose diet. As compared with mice, the hamster lipoprotein metabolism more closely resembles that of humans, and high-fructose diets raise plasma very low density lipoprotein (VLDL)-TG and cholesterol levels as well as hepatic TG content. A high-sucrose diet induces hepatic SREBP-1 and the mRNA levels of the genes required for fatty acid synthesis such as acetyl-CoA carboxylase and fatty acid synthase. Depletion of SCAP mRNA by RNAi in hamsters fed a high-sucrose diet for several weeks diminishes SCAP protein in the liver and nuclear SREBPs, which results in the normalization of mRNA levels of those genes. The inhibition of SREBP-1c activation by SCAP RNAi can normalize hepatic TG content as well as plasma VLDL-TG and VLDL-cholesterol in hamsters. The secretion rates of VLDL-TG are reduced by 40%; however, SCAP-RNAi does not significantly affect body weight, plasma insulin level, or blood glucose level in hamsters [14].
TARGETING SCAP IN ANIMAL MODELS OF INSULIN RESISTANCE
- In an insulin-resistant state, the increased influx of fatty acids from adipocytes, the increase in de novo lipogenesis, and reduced fatty acid oxidation result in TG accumulation in the liver. Activation of SREBP-1c, the main transcription factor that increases hepatic fatty acid and TG synthesis, is presented in insulin resistance. Inhibition of SCAP prevents the activation of SREBPs and the expression of genes required for fatty acid and TG synthesis, which results in the inhibition of de novo fatty acid synthesis. As a consequence, the inhibition of SCAP can resolve hepatic steatosis and hypertriglyceridemia in animal models of insulin resistance. Further human studies will reveal whether inhibition of SCAP has the same therapeutic effects in humans as it does in animal models.
CONCLUSIONS
-
Acknowledgements
- This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A 01059023).
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Article information
- 1. Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 2006;40(Suppl 1):S5–S10. PubMed
- 2. Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis 2008;28:339–350. ArticlePubMedPDF
- 3. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820–1832. ArticlePubMed
- 4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–1231. ArticlePubMed
- 5. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50:1844–1850. ArticlePubMed
- 6. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351. ArticlePubMedPMC
- 7. Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem 1999;274:30028–30032. ArticlePubMed
- 8. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002;109:1125–1131. ArticlePubMedPMC
- 9. Ishii S, Iizuka K, Miller BC, Uyeda K. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc Natl Acad Sci U S A 2004;101:15597–15602. ArticlePubMedPMC
- 10. Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 2003;278:34268–34276. ArticlePubMed
- 11. Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 2002;110:489–500. ArticlePubMed
- 12. Yabe D, Brown MS, Goldstein JL. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc Natl Acad Sci U S A 2002;99:12753–12758. ArticlePubMedPMC
- 13. Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest 1997;99:846–854. ArticlePubMedPMC
- 14. Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, et al. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab 2012;15:240–246. ArticlePubMedPMC
- 15. Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem 2002;277:9520–9528. ArticlePubMed
- 16. Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A 2004;101:7281–7286. ArticlePubMedPMC
- 17. Matsuda M, Korn BS, Hammer RE, Moon YA, Komuro R, Horton JD, et al. SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes Dev 2001;15:1206–1216. ArticlePubMedPMC
- 18. Bril F, Sninsky JJ, Baca AM, Superko HR, Portillo Sanchez P, Biernacki D, et al. Hepatic steatosis and insulin resistance, but not steatohepatitis, promote atherogenic dyslipidemia in NAFLD. J Clin Endocrinol Metab 2016;101:644–652. ArticlePubMed
- 19. Makadia SS, Blaha M, Keenan T, Ndumele C, Jones S, De-Filippis A, et al. Relation of hepatic steatosis to atherogenic dyslipidemia. Am J Cardiol 2013;112:1599–1604. ArticlePubMed
References
Fig. 1
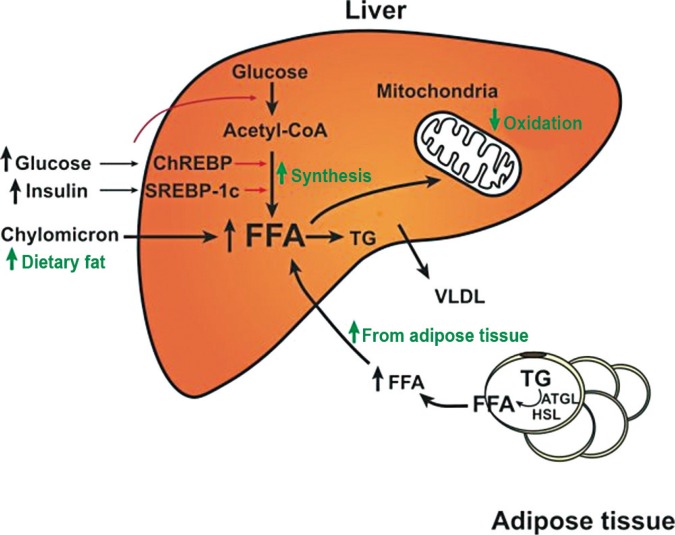
Metabolic alterations that cause hepatic steatosis in insulin-resistant states. Fatty acids in the liver are derived from diet, de novo synthesis, and peripheral adipose tissue. In insulin-resistant states, lipases in adipocytes are not inhibited by insulin, and free fatty acids (FFAs) are released continuously and taken up by hepatocytes. In the liver, hyperinsulinemia induces sterol regulatory element binding protein-1c (SREBP-1c) activity, which increases the de novo synthesis of fatty acids. Fatty acid oxidation in mitochondria is reduced due to the inhibition of carnitine palmitoyl transferase-1 by the malonyl-coenzyme A (CoA) generated from de novo fatty acid synthesis. Therefore, free fatty acids in the liver are preferentially esterified to triglycerides (TG). ChREBP, carbohydrate response element binding protein; VLDL, very low density lipoprotein; ATGL, adipose triglyceride lipase; HSL, hormone sensitive lipase.

Fig. 2
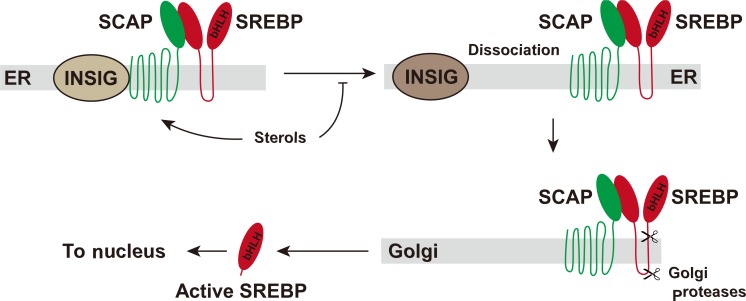
Activation of sterol regulatory element binding protein (SREBP). SREBPs are synthesized as inactive precursors that exist in the endoplasmic reticulum (ER) membrane. SREBP cleavage activating protein (SCAP) escorts SREBPs from the ER to the Golgi where two proteases release the NH2-teminal region of SREBPs. The NH2-teminal region of SREBPs moves to the nucleus and activates transcription of the target genes. INSIG, insulin induced gene; bHLH, basic helix-loop-helix.

Figure & Data
References
Citations
Citations to this article as recorded by 

- SREBPs as the potential target for solving the polypharmacy dilemma
Xue Wang, Yanqiu Chen, Heyu Meng, Fanbo Meng
Frontiers in Physiology.2024;[Epub] CrossRef - The Role of SCAP/SREBP as Central Regulators of Lipid Metabolism in Hepatic Steatosis
Preethi Chandrasekaran, Ralf Weiskirchen
International Journal of Molecular Sciences.2024; 25(2): 1109. CrossRef - Expression and correlation analysis of silent information regulator 1 (SIRT1), sterol regulatory element-binding protein-1 (SREBP1), and pyroptosis factor in gestational diabetes mellitus
Ning Han, Xin-yuan Chang, Zi-li Yuan, Yi-zhan Wang
The Journal of Maternal-Fetal & Neonatal Medicine.2024;[Epub] CrossRef - Uxi (Endopleura uchi (Huber) Cuatrec) bark extract mitigates HFD-induced adiposity in rats via targeting oxidative stress, and lipogenic genes expression
Eman A.R. Abdelghffar, Zuhair M. Mohammedsaleh, Raha Osailan, Aisha Elaimi, Wafae Ouchari, Mohamed A.O. Abdelfattah, Mona F. Mahmoud, Mansour Sobeh
Journal of Functional Foods.2024; 114: 106034. CrossRef - Robinetin Alleviates Metabolic Failure in Liver through Suppression of p300–CD38 Axis
Ji-Hye Song, Hyo-Jin Kim, Jangho Lee, Seung-Pyo Hong, Min-Yu Chung, Yu-Geun Lee, Jae Ho Park, Hyo-Kyoung Choi, Jin-Taek Hwang
Biomolecules & Therapeutics.2024; 32(2): 214. CrossRef - Regeneration of Non-Alcoholic Fatty Liver Cells Using Chimeric FGF21/HGFR: A Novel Therapeutic Approach
Sung-Jun Kim, So-Jung Kim, Jeongeun Hyun, Hae-Won Kim, Jun-Hyeog Jang
International Journal of Molecular Sciences.2024; 25(6): 3092. CrossRef - Evolutionary analysis of buffalo sterol regulatory element-binding factor (SREBF) family genes and their affection on milk traits
Tingzhu Ye, Jing Yuan, Sayed Haidar Abbas Raza, Tingxian Deng, Lv Yang, Muhammad Jamil Ahmad, Seyed Mahdi Hosseini, Xinxin Zhang, Muna O Alamoudi, Qwait AlGabbani, Youssef S Alghamdi, Chao Chen, Aixin Liang, Nicola M. Schreurs, Liguo Yang
Animal Biotechnology.2023; 34(7): 2082. CrossRef - Hepatic Pin1 Expression, Particularly in Nuclei, Is Increased in NASH Patients in Accordance with Evidence of the Role of Pin1 in Lipid Accumulation Shown in Hepatoma Cell Lines
Machi Kanna, Yusuke Nakatsu, Takeshi Yamamotoya, Akifumi Kushiyama, Midori Fujishiro, Hideyuki Sakoda, Hiraku Ono, Koji Arihiro, Tomoichiro Asano
International Journal of Molecular Sciences.2023; 24(10): 8847. CrossRef - Dietary acetate promotes growth and nutrients deposition in Nile tilapia (Oreochromis niloticus) through increasing acetyl-CoA-triggered energy production
Wen-Hao Zhou, Samwel M. Limbu, Yuan Luo, Rui-Xin Li, Jiong Ren, Fang Qiao, Mei-Ling Zhang, Zhen-Yu Du
Aquaculture.2023; 575: 739750. CrossRef - Palmitate로 유발된 비알코올성 지방간 세포 모델에서 블랙커런트 초임계 추출물의 지방증 개선효과
수정 이, 은숙 양, 영호 김, 혜연 김, 혜란 최, 석 김, 태호 류
Korean Journal of Food and Cookery Science.2023; 39(3): 178. CrossRef - PM2.5 induced liver lipid metabolic disorders in C57BL/6J mice
Chenxiao Zhang, Tengfei Ma, Chang Liu, Ding Ma, Jian Wang, Meng Liu, Jinjun Ran, Xueting Wang, Xiaobei Deng
Frontiers in Endocrinology.2023;[Epub] CrossRef - High Gamma-Aminobutyric Acid (GABA) Oolong Tea Alleviates High-Fat Diet-Induced Metabolic Disorders in Mice
Monthana Weerawatanakorn, Sang He, Chun-Han Chang, Yen-Chun Koh, Meei-Ju Yang, Min-Hsiung Pan
ACS Omega.2023; 8(37): 33997. CrossRef - MicroRNA-19a regulates milk fat metabolism by targeting SYT1 in bovine mammary epithelial cells
Baojun Yu, Jiamin Liu, Zhengyun Cai, Tong Mu, Di Zhang, Xiaofang Feng, Yaling Gu, Juan Zhang
International Journal of Biological Macromolecules.2023; 253: 127096. CrossRef - SREBP Regulation of Lipid Metabolism in Liver Disease, and Therapeutic Strategies
Na Li, Xiaodan Li, Yifu Ding, Xiao Liu, Karin Diggle, Tatiana Kisseleva, David A. Brenner
Biomedicines.2023; 11(12): 3280. CrossRef - Daidzein stimulates fatty acid-induced fat deposition in C2C12 myoblast cells via the G protein-coupled receptor 30 pathway
Chengjian Zhou, Ping Li, Meihong Han, Xuejun Gao
Animal Biotechnology.2022; 33(5): 851. CrossRef - Hepatocyte steatosis inhibits hepatitis B virus secretion via induction of endoplasmic reticulum stress
Qichuang Liu, Maoyuan Mu, Huan Chen, Guoyuan Zhang, Yanqing Yang, Jun Chu, Ying Li, Fangwan Yang, Shide Lin
Molecular and Cellular Biochemistry.2022; 477(11): 2481. CrossRef - The cholesterol pathway: impact on immunity and cancer
Ryan J. King, Pankaj K. Singh, Kamiya Mehla
Trends in Immunology.2022; 43(1): 78. CrossRef - Hepatic retinaldehyde dehydrogenases are modulated by tocopherol supplementation in mice with hepatic steatosis
Amanda D'Espessailles, Valeria Campos, Nevenka Juretić, Gladys S. Tapia, Paulina Pettinelli
Nutrition.2022; 94: 111539. CrossRef - CD36 promotes de novo lipogenesis in hepatocytes through INSIG2-dependent SREBP1 processing
Han Zeng, Hong Qin, Meng Liao, Enze Zheng, Xiaoqing Luo, Anhua Xiao, Yiyu Li, Lin Chen, Li Wei, Lei Zhao, Xiong Z. Ruan, Ping Yang, Yaxi Chen
Molecular Metabolism.2022; 57: 101428. CrossRef - Biogenesis and Breakdown of Lipid Droplets in Pathological Conditions
Claudio M. Fader Kaiser, Patricia S. Romano, M. Cristina Vanrell, Cristian A. Pocognoni, Julieta Jacob, Benjamín Caruso, Laura R. Delgui
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - Efficacy and safety of an orally administered DGAT2 inhibitor alone or coadministered with a liver-targeted ACC inhibitor in adults with non-alcoholic steatohepatitis (NASH): rationale and design of the phase II, dose-ranging, dose-finding, randomised, pl
Neeta B Amin, Amanda Darekar, Quentin M Anstee, Vincent Wai-Sun Wong, Frank Tacke, Manoli Vourvahis, Douglas S Lee, Michael Charlton, Naim Alkhouri, Atsushi Nakajima, Carla Yunis
BMJ Open.2022; 12(3): e056159. CrossRef - Identification of microRNA Transcriptome Involved in Bovine Intramuscular Fat Deposition
Susan K. Duckett, Maslyn A. Greene
Frontiers in Veterinary Science.2022;[Epub] CrossRef - Camellia (Camellia oleifera bel.) seed oil reprograms gut microbiota and alleviates lipid accumulation in high fat-fed mice through the mTOR pathway
Jing Gao, Li Ma, Jie Yin, Gang Liu, Jie Ma, SiTing Xia, SaiMing Gong, Qi Han, TieJun Li, YongZhong Chen, YuLong Yin
Food & Function.2022; 13(9): 4977. CrossRef - The Perirenal Fat Thickness Was Associated with Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus
Yuxian Yang, Shuting Li, Yuechao Xu, Jing Ke, Dong Zhao
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2022; Volume 15: 1505. CrossRef - Vanadium(IV)-Chlorodipicolinate Protects against Hepatic Steatosis by Ameliorating Lipid Peroxidation, Endoplasmic Reticulum Stress, and Inflammation
Yuanli Wang, Rulong Chen, Jingyi Li, Guodong Zeng, Juntao Yuan, Jingran Su, Chunyan Wu, Zhongbing Lu, Fang Zhang, Wenjun Ding
Antioxidants.2022; 11(6): 1093. CrossRef - Inhibiting SCAP/SREBP exacerbates liver injury and carcinogenesis in murine nonalcoholic steatohepatitis
Satoshi Kawamura, Yuki Matsushita, Shigeyuki Kurosaki, Mizuki Tange, Naoto Fujiwara, Yuki Hayata, Yoku Hayakawa, Nobumi Suzuki, Masahiro Hata, Mayo Tsuboi, Takahiro Kishikawa, Hiroto Kinoshita, Takuma Nakatsuka, Masaya Sato, Yotaro Kudo, Yujin Hoshida, At
Journal of Clinical Investigation.2022;[Epub] CrossRef - Baicalein Prevents Fructose-Induced Hepatic Steatosis in Rats: In the Regulation of Fatty Acid De Novo Synthesis, Fatty Acid Elongation and Fatty Acid Oxidation
Pan Li, Ruoyu Zhang, Meng Wang, Yuwei Chen, Zhiwei Chen, Xiumei Ke, Ling Zuo, Jianwei Wang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Identification and validation of immune related core transcription factors GTF2I in NAFLD
Minbo Zhang, Yu Zhang, Xiaoxiao Jiao, Linying Lai, Yiting Qian, Bo Sun, Wenzhuo Yang
PeerJ.2022; 10: e13735. CrossRef - Lipid-lowering effect of microencapsulated peptides from brewer's spent grain in high-sucrose diet-fed rats
M.R. Ferreira, A.G. Garzón, M.E. Oliva, R.E. Cian, S.R. Drago, M.E. D'Alessandro
Food Bioscience.2022; 49: 101981. CrossRef - The SREBP-dependent regulation of cyclin D1 coordinates cell proliferation and lipid synthesis
Arwa Aldaalis, Maria T. Bengoechea-Alonso, Johan Ericsson
Frontiers in Oncology.2022;[Epub] CrossRef - Targets of statins intervention in LDL-C metabolism: Gut microbiota
ChangXin Sun, ZePing Wang, LanQing Hu, XiaoNan Zhang, JiYe Chen, ZongLiang Yu, LongTao Liu, Min Wu
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Regulation of lipid droplet (LD) formation in hepatocytes via regulation of SREBP1c by non-coding RNAs
Shereen A. El Sobky, Nourhan K. Aboud, Nihal M. El Assaly, Injie O. Fawzy, Nada El-Ekiaby, Ahmed I. Abdelaziz
Frontiers in Medicine.2022;[Epub] CrossRef - Aspalathin-rich green rooibos tea in combination with glyburide and atorvastatin enhances lipid metabolism in a db/db mouse model
Oelfah Patel, Christo J. F. Muller, Elizabeth Joubert, Bernd Rosenkranz, Johan Louw, Charles Awortwe
Frontiers in Clinical Diabetes and Healthcare.2022;[Epub] CrossRef - Capparis spinosa improves non-alcoholic steatohepatitis through down-regulating SREBP-1c and a PPARα-independent pathway in high-fat diet-fed rats
Rasoul Akbari, Hamid Yaghooti, Mohammad Taha Jalali, Laya Sadat Khorsandi, Narges Mohammadtaghvaei
BMC Research Notes.2022;[Epub] CrossRef - Gypenosides counteract hepatic steatosis and intestinal barrier injury in rats with metabolic associated fatty liver disease by modulating the adenosine monophosphate activated protein kinase and Toll-like receptor 4/nuclear factor kappa B pathways
Shuhua Shen, Kungen Wang, Yihui Zhi, Yue Dong
Pharmaceutical Biology.2022; 60(1): 1949. CrossRef - CYP51-mediated cholesterol biosynthesis is required for the proliferation of CD4+ T cells in Sjogren’s syndrome
Junhao Yin, Jiayao Fu, Yanxiong Shao, Jiabao Xu, Hui Li, Changyu Chen, Yijie Zhao, Zhanglong Zheng, Chuangqi Yu, Lingyan Zheng, Baoli Wang
Clinical and Experimental Medicine.2022; 23(5): 1691. CrossRef - Differential TM4SF5‐mediated SIRT1 modulation and metabolic signaling in nonalcoholic steatohepatitis progression
Jihye Ryu, Eunmi Kim, Min‐Kyung Kang, Dae‐Geun Song, Eun‐Ae Shin, Haesong Lee, Jae Woo Jung, Seo Hee Nam, Ji Eon Kim, Hye‐Jin Kim, Taekwon Son, Semi Kim, Hwi Young Kim, Jung Weon Lee
The Journal of Pathology.2021; 253(1): 55. CrossRef - Changes in Glutathione Content in Liver Diseases: An Update
Mariapia Vairetti, Laura Giuseppina Di Pasqua, Marta Cagna, Plinio Richelmi, Andrea Ferrigno, Clarissa Berardo
Antioxidants.2021; 10(3): 364. CrossRef - Metreleptin therapy for nonalcoholic steatohepatitis: Open-label therapy interventions in two different clinical settings
Baris Akinci, Angela Subauste, Nevin Ajluni, Nazanene H. Esfandiari, Rasimcan Meral, Adam H. Neidert, Akin Eraslan, Rita Hench, Diana Rus, Barbara Mckenna, Hero K. Hussain, Thomas L. Chenevert, Marwan K. Tayeh, Amit R. Rupani, Jeffrey W. Innis, Christos S
Med.2021; 2(7): 814. CrossRef - Prediction of Srebp-1 as a Key Target of Qing Gan San Against MAFLD in Rats via RNA-Sequencing Profile Analysis
Bendong Yang, Jingyue Sun, Shufei Liang, Peixuan Wu, Rui Lv, Yanping He, Deqi Li, Wenlong Sun, Xinhua Song
Frontiers in Pharmacology.2021;[Epub] CrossRef - Structural insights into the mechanism of human NPC1L1-mediated cholesterol uptake
Miaoqing Hu, Fan Yang, Yawen Huang, Xin You, Desheng Liu, Shan Sun, Sen-Fang Sui
Science Advances.2021;[Epub] CrossRef - Identification and Optimization of a Minor Allele-Specific siRNA to Prevent PNPLA3 I148M-Driven Nonalcoholic Fatty Liver Disease
Justin K. Murray, Jason Long, Lei Liu, Shivani Singh, Danielle Pruitt, Michael Ollmann, Elissa Swearingen, Miki Hardy, Oliver Homann, Bin Wu, Jerry Ryan Holder, Kelvin Sham, Brad Herberich, Mei-Chu Lo, Hui Dou, Artem Shkumatov, Monica Florio, Ingrid C. Ru
Nucleic Acid Therapeutics.2021; 31(5): 324. CrossRef - Molecular mechanism of DLBS3733, a bioactive fraction of Lagerstroemia speciosa (L.) Pers., on ameliorating hepatic lipid accumulation in HepG2 cells
Olivia M. Tandrasasmita, Guntur Berlian, Raymond R. Tjandrawinata
Biomedicine & Pharmacotherapy.2021; 141: 111937. CrossRef - The Role of Gut Microbiota on Cholesterol Metabolism in Atherosclerosis
Margaret Vourakis, Gaétan Mayer, Guy Rousseau
International Journal of Molecular Sciences.2021; 22(15): 8074. CrossRef - Inhibition of Atherosclerosis and Liver Steatosis by Agmatine in Western Diet-Fed apoE-Knockout Mice Is Associated with Decrease in Hepatic De Novo Lipogenesis and Reduction in Plasma Triglyceride/High-Density Lipoprotein Cholesterol Ratio
Anna Wiśniewska, Aneta Stachowicz, Katarzyna Kuś, Magdalena Ulatowska-Białas, Justyna Totoń-Żurańska, Anna Kiepura, Kamila Stachyra, Maciej Suski, Mariusz Gajda, Jacek Jawień, Rafał Olszanecki
International Journal of Molecular Sciences.2021; 22(19): 10688. CrossRef - Therapeutic Targeting of Nonalcoholic Fatty Liver Disease by Downregulating SREBP-1C Expression via AMPK-KLF10 Axis
Yu-Chi Chen, Rong-Jane Chen, Szu-Yuan Peng, Winston C. Y. Yu, Vincent Hung-Shu Chang
Frontiers in Molecular Biosciences.2021;[Epub] CrossRef - Regulation of Key Genes for Milk Fat Synthesis in Ruminants
Tong Mu, Honghong Hu, Yanfen Ma, Xiaofang Feng, Juan Zhang, Yaling Gu
Frontiers in Nutrition.2021;[Epub] CrossRef - Visceral-to-Subcutaneous Abdominal Fat Ratio Is Associated with Nonalcoholic Fatty Liver Disease and Liver Fibrosis
Chan-Hee Jung, Eun-Jung Rhee, Hyemi Kwon, Yoosoo Chang, Seungho Ryu, Won-Young Lee
Endocrinology and Metabolism.2020; 35(1): 165. CrossRef - Lipidomic profiling analysis of the phospholipid molecules in SCAP-induced lipid droplet formation in bovine mammary epithelial cells
Liqiang Han, Kun Pang, Xiu ling Li, Juan J Loor, Guo yu Yang, Tengyun Gao
Prostaglandins & Other Lipid Mediators.2020; 149: 106420. CrossRef - Sexual Dimorphism of NAFLD in Adults. Focus on Clinical Aspects and Implications for Practice and Translational Research
Amedeo Lonardo, Ayako Suzuki
Journal of Clinical Medicine.2020; 9(5): 1278. CrossRef - Smurf1 aggravates non‐alcoholic fatty liver disease by stabilizing SREBP‐1c in an E3 activity‐independent manner
Xin Zhang, Yutao Zhan, Wenjun Lin, Fei Zhao, Chaojing Guo, Yujiao Chen, Mengge Du, Dongnian Li, Lingqiang Zhang, Wei An, Hong‐Rui Wang, Ping Xie
The FASEB Journal.2020; 34(6): 7631. CrossRef - Beneficial Effects of SREBP Decoy Oligodeoxynucleotide in an Animal Model of Hyperlipidemia
Hyun-Jin An, Jung-Yeon Kim, Mi-Gyeong Gwon, Hyemin Gu, Hyun-Ju Kim, Jaechan Leem, Sung Won Youn, Kwan-Kyu Park
International Journal of Molecular Sciences.2020; 21(2): 552. CrossRef - Lack of Augmenter of Liver Regeneration Disrupts Cholesterol Homeostasis of Liver in Mice by Inhibiting the AMPK Pathway
Xin Wang, Ling‐yue Dong, Qu‐jing Gai, Wei‐lun Ai, Yuan Wu, Wei‐chun Xiao, Jing Zhang, Wei An
Hepatology Communications.2020; 4(8): 1149. CrossRef - HESA-A Attenuates Hepatic Steatosis in NAFLD Rat Model Through the Suppression of SREBP-1c and NF-kβ
M. Efati, M. Khorrami, Z. Jangravi, A. Z. Mahmoudabadi, M. Raeiszadeh, J. R. Sarshoori
International Journal of Peptide Research and Therapeutics.2020; 26(3): 1283. CrossRef - Prevention of Nonalcoholic Hepatic Steatosis by Shenling Baizhu Powder: Involvement of Adiponectin-Induced Inhibition of Hepatic SREBP-1c
Kairui Tang, Yuanjun Deng, Chuiyang Zheng, Huan Nie, Maoxing Pan, Runsen Chen, Jiqian Xie, Qinhe Yang, Yupei Zhang
Oxidative Medicine and Cellular Longevity.2020; 2020: 1. CrossRef - (−)-Epicatechin and the comorbidities of obesity
Eleonora Cremonini, Dario E. Iglesias, Jiye Kang, Giovanni E. Lombardo, Zahra Mostofinejad, Ziwei Wang, Wei Zhu, Patricia I. Oteiza
Archives of Biochemistry and Biophysics.2020; 690: 108505. CrossRef - microRNAs targeting cellular cholesterol: implications for combating anticancer drug resistance
Bernice Monchusi, Mandeep Kaur
Genes & Cancer.2020; 11(1-2): 20. CrossRef - Cellular acidosis triggers human MondoA transcriptional activity by driving mitochondrial ATP production
Blake R Wilde, Zhizhou Ye, Tian-Yeh Lim, Donald E Ayer
eLife.2019;[Epub] CrossRef - Differential Recovery Speed of Activity and Metabolic Rhythms in Rats After an Experimental Protocol of Shift-Work
Nadia Saderi, Adrián Báez-Ruiz, Lucia E. Azuara-Álvarez, Carolina Escobar, Roberto C. Salgado-Delgado
Journal of Biological Rhythms.2019; 34(2): 154. CrossRef - Genetically modified mouse models to study hepatic neutral lipid mobilization
Guenter Haemmerle, Achim Lass
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2019; 1865(5): 879. CrossRef - Preventive effect of trans-chalcone on non-alcoholic steatohepatitis: Improvement of hepatic lipid metabolism
Elham Karimi-Sales, Abbas Ebrahimi-Kalan, Mohammad Reza Alipour
Biomedicine & Pharmacotherapy.2019; 109: 1306. CrossRef - A new pathway to eSCAPe lipotoxicity
Fadila Benhamed, Catherine Postic
Clinics and Research in Hepatology and Gastroenterology.2018; 42(1): 3. CrossRef - Overexpression of SREBF chaperone (SCAP) enhances nuclear SREBP1 translocation to upregulate fatty acid synthase (FASN) gene expression in bovine mammary epithelial cells
L.Q. Han, T.Y. Gao, G.Y. Yang, J.J. Loor
Journal of Dairy Science.2018; 101(7): 6523. CrossRef - Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study
Gyuri Kim, Suk-Yong Jang, Chung Mo Nam, Eun Seok Kang
Journal of Hepatology.2018; 68(3): 476. CrossRef - Changes of the Fatty Acid Profile in Erythrocyte Membranes of Patients following 6-Month Dietary Intervention Aimed at the Regression of Nonalcoholic Fatty Liver Disease (NAFLD)
Dominika Maciejewska, Wojciech Marlicz, Karina Ryterska, Marcin Banaszczak, Dominika Jamioł-Milc, Ewa Stachowska
Canadian Journal of Gastroenterology and Hepatology.2018; 2018: 1. CrossRef - Systems Analysis of the Liver Transcriptome in Adult Male Zebrafish Exposed to the Plasticizer (2-Ethylhexyl) Phthalate (DEHP)
Matthew Huff, Willian A. da Silveira, Oliana Carnevali, Ludivine Renaud, Gary Hardiman
Scientific Reports.2018;[Epub] CrossRef - Inactivation of ceramide synthase 2 catalytic activity in mice affects transcription of genes involved in lipid metabolism and cell division
Andreas Bickert, Paul Kern, Martina van Uelft, Stefanie Herresthal, Thomas Ulas, Katharina Gutbrod, Bernadette Breiden, Joachim Degen, Konrad Sandhoff, Joachim L. Schultze, Peter Dörmann, Dieter Hartmann, Reinhard Bauer, Klaus Willecke
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2018; 1863(7): 734. CrossRef - Paradoxical Protective Effect of Perfluorooctanesulfonic Acid Against High-Fat Diet–Induced Hepatic Steatosis in Mice
Ian Huck, Kevin Beggs, Udayan Apte
International Journal of Toxicology.2018; 37(5): 383. CrossRef - Developmental and extrahepatic physiological functions of SREBP pathway genes in mice
Luke J. Engelking, Mary Jo Cantoria, Yanchao Xu, Guosheng Liang
Seminars in Cell & Developmental Biology.2018; 81: 98. CrossRef - Herbacetin, a flaxseed flavonoid, ameliorates high percent dietary fat induced insulin resistance and lipid accumulation through the regulation of hepatic lipid metabolizing and lipid-regulating enzymes
Chinnadurai Veeramani, Mohammed A. Alsaif, Khalid S. Al-Numair
Chemico-Biological Interactions.2018; 288: 49. CrossRef - Isocaloric Dietary Changes and Non-Alcoholic Fatty Liver Disease in High Cardiometabolic Risk Individuals
Giuseppe Della Pepa, Claudia Vetrani, Gianluca Lombardi, Lutgarda Bozzetto, Giovanni Annuzzi, Angela Rivellese
Nutrients.2017; 9(10): 1065. CrossRef - MDG-1, a Potential Regulator of PPARα and PPARγ, Ameliorates Dyslipidemia in Mice
Xu Wang, Linlin Shi, Sun Joyce, Yuan Wang, Yi Feng
International Journal of Molecular Sciences.2017; 18(9): 1930. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite



