Articles
- Page Path
- HOME > Endocrinol Metab > Volume 31(1); 2016 > Article
-
Namgok Lecture 2015The Impact of Organokines on Insulin Resistance, Inflammation, and Atherosclerosis
-
Kyung Mook Choi

-
Endocrinology and Metabolism 2016;31(1):1-6.
DOI: https://doi.org/10.3803/EnM.2016.31.1.1
Published online: March 16, 2016
Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- Corresponding author: Kyung Mook Choi. Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, 148 Gurodong-ro, Guro-gu, Seoul 08308, Korea. Tel: +82-2-2626-3043, Fax: +82-2-2626-1096, medica7@gmail.com
Copyright © 2016 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Immoderate energy intake, a sedentary lifestyle, and aging have contributed to the increased prevalence of obesity, sarcopenia, metabolic syndrome, type 2 diabetes, and cardiovascular disease. There is an urgent need for the development of novel pharmacological interventions that can target excessive fat accumulation and decreased muscle mass and/or strength. Adipokines, bioactive molecules derived from adipose tissue, are involved in the regulation of appetite and satiety, inflammation, energy expenditure, insulin resistance and secretion, glucose and lipid metabolism, and atherosclerosis. Recently, there is emerging evidence that skeletal muscle and the liver also function as endocrine organs that secrete myokines and hepatokines, respectively. Novel discoveries and research into these organokines (adipokines, myokines, and hepatokines) may lead to the development of promising biomarkers and therapeutics for cardiometabolic disease. In this review, I summarize recent data on these organokines and focus on the role of adipokines, myokines, and hepatokines in the regulation of insulin resistance, inflammation, and atherosclerosis.
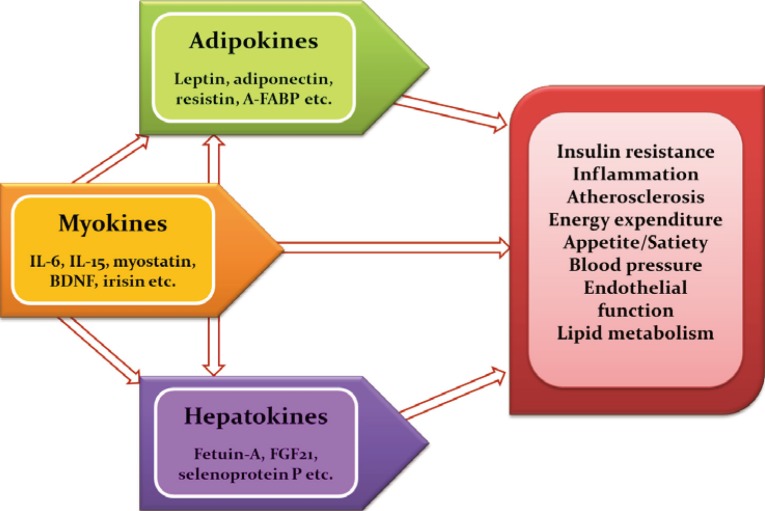
- Obesity significantly increases the risk of cardiometabolic diseases, such as type 2 diabetes, dyslipidemia, hypertension, coronary heart disease, and stroke. Furthermore, recent evidence has shown that obesity is a risk factor for nonalcoholic fatty liver disease (NAFLD), obstructive sleep apnea, dementia, and several types of cancers [1]. The main known function of adipose tissue is to store excess calories in the form of triglycerides and to release them during fasting and cold exposure. Moreover, adipose tissue has been established to be an endocrine organ that secretes adipokines, which are bioactive peptides that control systemic metabolism and energy homeostasis [2]. Organokines, which are predominantly produced and se-creted by their respective tissues, affect whole body metabolism through autocrine, paracrine, and endocrine activities (Fig. 1). Autologous to adipokines, myokines from skeletal muscle and hepatokines from the liver also regulate a variety of biological processes and communicate with distant target organs.
INTRODUCTION
- In obese individuals, the hypertrophy of adipocytes predisposes to immune cell infiltration, which produces proinflammatory cytokines, including interleukin 6 (IL-6), IL-8, and monocyte chemoattractant protein-1 (MCP-1). With increasing body weight, individuals develop adipocyte hypertrophy, hypoxia, and ectopic fat deposition, which induces the altered production and secretion of adipokines in adipose tissue [3]. Adipose tissue dysfunction and disturbed adipokine secretion may connect obesity with its metabolic, inflammatory, and cardiovascular complications [45]. Adipokines such as leptin, adiponectin, tumor necrosis factor α (TNF-α), retinol binding protein 4 (RBP4), adipocyte fatty acid binding protein (A-FABP), resistin, vaspin, apelin, chemerin, omentin, and C1q/TNF-related proteins (CTRPs) are involved in a spectrum of obesity-associated disorders. In fact, several adipokines, such as leptin, RBP4, IL-6, chemerin, and progranulin, are differentially expressed according to fat deposition [1].
- Leptin is a prototype adipokine discovered in 1994 [6]. Leptin has a pivotal role in the regulation of appetite, satiety, food intake, energy expenditure, reproductive function, and fertility [7]. In the hypothalamus, leptin decreases orexigenic- and increases anorexigenic-neuropeptide synthesis, resulting in reduced appetite and body weight in animal experiments [8]. However, circulating leptin levels are proportional to body fat mass, and treatment with recombinant leptin shows only small effects on weight loss in humans [9]. These results support the concept of leptin resistance or tolerance in obese individuals. Adiponectin is a 30-kDa protein which signals via at least two adiponectin receptors, AdipoR1 and AdipoR2 [10]. Post-translational modification of adiponectin results in 180-kDa hexamers (low molecular weight) and 18- to 36-mers (high molecular weight) [1011]. Adiponectin improves insulin sensitivity and has anti-diabetic, anti-inflammatory, and anti-atherogenic properties [1]. Circulating adiponectin levels are inversely associated with components of the metabolic syndrome, including body weight, blood pressure, lipids, and insulin resistance. Furthermore, adiponectin levels show a negative relationship with visceral fat, type 2 diabetes, and cardiovascular disease (CVD) [11]. We reported that circulating adiponectin levels showed a significant negative correlation with the mean target-to-background ratio, which reflects vascular inflammation using 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) [12]. A-FABP is a novel adipokine that plays a key role in linking obesity with various features of metabolic syndrome, type 2 diabetes, and CVD [1314]. In macrophages, A-FABP potentiates toxic lipid-induced inflammation and endoplasmic reticulum (ER) stress, which may be involved in the pathogenesis of CVD [15]. Several prospective studies have shown that high A-FBAP concentrations at baseline are predictive of the risk for metabolic and vascular morbidity, as well as mortality [16]. In our previous study, A-FABP levels predicted the development of metabolic syndrome independent of traditional risk factors in Korean children [17]. Recent studies have shown that the CTRP family of proteins, paralogs of adiponectin, play a crucial role in the regulation of systemic metabolism and innate immunity [1819]. CTRP-1 to CTRP-15 show remarkable similarities in their structural and biochemical properties compared to adiponectin [18]. We observed that CTRP-3 concentrations are elevated in patients with dysregulation of glucose metabolism and are associated with various metabolic risk factors [20]. Moreover, a 3-month combined exercise program significantly affected CTPR-3 and CTRP-5 levels in obese Korean women [21]. Patients with acute coronary syndrome and stable angina pectoris had significantly lower circulating CTRP-3 levels compared to control subjects [22]. Recently, we demonstrated that CTRP9 attenuates hepatic steatosis through the alleviation of ER stress via the AMP-activated protein kinase (AMPK)-mediated induction of autophagy [23].
ADIPOKINES
- Aging and physical inactivity result in a progressive loss of muscle mass and strength, known as sarcopenia. We have previously studied the prevalence of sarcopenia and its impact on metabolic disorders, including type 2 diabetes and NAFLD [242526]. Regular exercise is an effective way to prevent and treat chronic metabolic disorders such as type 2 diabetes and metabolic syndrome. Myokines, proteins that are released from skeletal muscle, may mediate the beneficial effects of regular physical activity [27]. In fact, exercise affects the expression in skeletal muscle and the circulating levels of a number of myokines, such as IL-6, IL-15, angiopoietin-like 4, myostatin, and irisin [28].
- IL-6 is the first myokine that has been known to be a proinflammatory cytokine. Dr. Pedersen's group first demonstrated that IL-6 mRNA expression in muscle, as well as circulating levels of IL-6, were markedly increased after exercise [29]. During acute exercise, IL-6 improves insulin sensitivity by blocking proinflammatory signaling pathways in the muscle [30]. However, chronically elevated IL-6 induces insulin resistance in adipose tissue and the liver [27]. Irisin is an exercise-induced novel myokine that promotes the browning of white adipose tissue and may mediate the health-promoting effects of exercise, including improvement in glycemic control [31]. However, controversy has arisen regarding inconsistencies in the regulation of irisin after exercise [32]. We showed that circulating irisin concentrations were not different in individuals with sarcopenia and those with brown adipose tissue detectable by 18FDG-PET [33].
MYOKINES
- The liver is a major regulator of systemic metabolism and whole body energy homeostasis. Liver-derived proteins known as hepatokines have recently emerged as novel hormones that have ambivalent roles, either aggravating insulin resistance or improving metabolic variables in the metabolic syndrome [34]. Fetuin-A is the first-known hepatokine that integrates crosstalk between the liver and target organs. Fetuin-A, a natural inhibitor of the insulin-stimulated insulin receptor tyrosine kinase, induces insulin resistance in rodents [35]. Fetuin-A was identified as an endogenous ligand for Toll-like receptor 4 through which saturated fatty acids induce proinflammatory signaling and insulin resistance [36]. Circulating fetuin-A levels are increased in obesity, metabolic syndrome, and type 2 diabetes, and are correlated with hepatic steatosis in humans [37]. Furthermore, prospective studies have shown that fetuin-A levels predict the increased risk of type 2 diabetes as well as myocardial infarction and stroke [3839]. We reported that adiponectin and salsalate improve hepatic steatosis by the inhibition of fetuin-A through the AMPK-nuclear factor-κB pathway [40]. Furthermore, we found that caloric restriction significantly decreased hepatic fetuin-A expression and its circulating levels in obese rats and humans with type 2 diabetes [41]. Fibroblast growth factor 21 (FGF21) is a central metabolic regulator that has favorable effects on glucose and lipid metabolism [42]. FGF21 is upregulated by the nuclear receptor peroxisome proliferator-activated receptor α during starvation [43]. Administration of FGF21 has been shown to decrease body weight, blood glucose, and lipid levels, as well as improves insulin resistance in animal experiments [44]. FGF21 decreases blood glucose levels by increasing glucose transporter 1 expression in human primary adipocytes and reduces triglyceride levels by inhibiting lipolysis and augmenting β-oxidation in the liver and adipose tissue [4546]. Circulating concentrations and the hepatic expression of FGF21 are increased in patients with NAFLD [47]. In a recent randomized clinical trial, treatment with LY2405319, an analog of FGF21, resulted in significant improvements in the dyslipidemia of obese human subjects with type 2 diabetes [48]. Selenoprotein P is positively correlated with insulin resistance and is upregulated in patients with type 2 diabetes [49]. In our previous study, selenoprotein P levels were elevated in patients with dysregulated glucose metabolism and were significantly associated with insulin resistance, inflammation, and carotid intima-media thickness [50]. We also reported that AMPK activators alleviate carrageenan-induced insulin resistance through the AMPK-mediated amelioration of ER stress in hepatocytes [51]. Circulating selenoprotein P levels are negatively correlated with adiponectin levels in patients with type 2 diabetes, suggesting the existence of crosstalk between a hepatokine (selenoprotein P) and an adipokine (adiponectin) [52].
HEPATOKINES
- Adipose tissue is now established as an active endocrine organ that secretes adipokines. Altered adipokine production and secretion may provide a link between adipose tissue dysfunction and obesity-related disorders. Adipokines are pivotal regulators in whole body metabolism because they are involved in impaired insulin sensitivity or secretion, inflammation, fat distribution, appetite, and satiety, as well as endothelial dysfunction and atherosclerosis. Analogous to the role of adipokines, myokines and hepatokines have also been proven to have crucial pathogenic roles in metabolic syndrome, NAFLD, type 2 diabetes, and CVD. The identification and functional characterization of novel organokines may provide important insights that could lead to novel treatments for cardiometabolic diseases.
CONCLUSIONS
-
Acknowledgements
- I would like to express my sincere gratitude to emeritus professor Hun Ki Min (Namgok) for inviting me to present the Namgok lecture and to professor Sei Hyun Baik and professor Dong Seop Choi for their mentorship. This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI14C0133) (K.M.C.).
ACKNOWLEDGMENTS
-
Namgok Award is the highest scientific award by the Korean Endocrine Society and honors to an individual who has excellently contributed to the progress in the field of endocrinology and metabolism. Namgok Award is named after the pen name of Professor Hunki Min, who has founded the Korean Endocrine Society in 1982.
Prof. Kyung Mook Choi has received Namgok Award at Autumn Symposium of the Korean Endocrine Society in November, 2015.
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Article information
- 1. Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci 2015;36:461–470. ArticlePubMed
- 2. Bluher M. Clinical relevance of adipokines. Diabetes Metab J 2012;36:317–327. ArticlePubMedPMC
- 3. Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord 2014;15:277–287. ArticlePubMedPDF
- 4. Yoo HJ, Choi KM. Adipokines as a novel link between obesity and atherosclerosis. World J Diabetes 2014;5:357–363. ArticlePubMedPMC
- 5. Oh JY. Regional adiposity, adipokines, and insulin resistance in type 2 diabetes. Diabetes Metab J 2012;36:412–414. ArticlePubMedPMC
- 6. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432. ArticlePubMedPDF
- 7. Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab 2011;301:E567–E584. ArticlePubMedPMC
- 8. Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism 2015;64:131–145. ArticlePubMed
- 9. Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 1999;282:1568–1575. ArticlePubMed
- 10. Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab 2013;2:133–141. ArticlePubMedPMC
- 11. Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia 2012;55:2319–2326. ArticlePubMedPDF
- 12. Choi HY, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, et al. Association of adiponectin, resistin, and vascular inflammation: analysis with 18F-fluorodeoxyglucose positron emission tomography. Arterioscler Thromb Vasc Biol 2011;31:944–949. ArticlePubMed
- 13. Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab 2005;1:107–119. ArticlePubMed
- 14. Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem 2006;52:405–413. ArticlePubMedPDF
- 15. Xu A, Vanhoutte PM. Adiponectin and adipocyte fatty acid binding protein in the pathogenesis of cardiovascular disease. Am J Physiol Heart Circ Physiol 2012;302:H1231–H1240. ArticlePubMed
- 16. Kralisch S, Fasshauer M. Adipocyte fatty acid binding protein: a novel adipokine involved in the pathogenesis of metabolic and vascular disease? Diabetologia 2013;56:10–21. ArticlePubMedPDF
- 17. Choi KM, Yannakoulia M, Park MS, Cho GJ, Kim JH, Lee SH, et al. Serum adipocyte fatty acid-binding protein, retinol-binding protein 4, and adiponectin concentrations in relation to the development of the metabolic syndrome in Korean boys: a 3-y prospective cohort study. Am J Clin Nutr 2011;93:19–26. ArticlePubMedPDF
- 18. Seldin MM, Tan SY, Wong GW. Metabolic function of the CTRP family of hormones. Rev Endocr Metab Disord 2014;15:111–123. ArticlePubMedPMCPDF
- 19. Schaffler A, Buechler C. CTRP family: linking immunity to metabolism. Trends Endocrinol Metab 2012;23:194–204. ArticlePubMed
- 20. Choi KM, Hwang SY, Hong HC, Yang SJ, Choi HY, Yoo HJ, et al. C1q/TNF-related protein-3 (CTRP-3) and pigment epithelium-derived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes 2012;61:2932–2936. ArticlePubMedPMC
- 21. Choi HY, Park JW, Lee N, Hwang SY, Cho GJ, Hong HC, et al. Effects of a combined aerobic and resistance exercise program on C1q/TNF-related protein-3 (CTRP-3) and CTRP-5 levels. Diabetes Care 2013;36:3321–3327. ArticlePubMedPMC
- 22. Choi KM, Hwang SY, Hong HC, Choi HY, Yoo HJ, Youn BS, et al. Implications of C1q/TNF-related protein-3 (CTRP-3) and progranulin in patients with acute coronary syndrome and stable angina pectoris. Cardiovasc Diabetol 2014;13:14ArticlePubMedPMC
- 23. Jung TW, Hong HC, Hwang HJ, Yoo HJ, Baik SH, Choi KM. C1q/TNF-related protein 9 (CTRP9) attenuates hepatic steatosis via the autophagy-mediated inhibition of endoplasmic reticulum stress. Mol Cell Endocrinol 2015;417:131–140. ArticlePubMed
- 24. Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond) 2009;33:885–892. ArticlePubMedPDF
- 25. Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010;33:1497–1499. ArticlePubMedPMC
- 26. Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 2014;59:1772–1778. ArticlePubMed
- 27. Catoire M, Kersten S. The search for exercise factors in humans. FASEB J 2015;29:1615–1628. ArticlePubMed
- 28. Eckardt K, Gorgens SW, Raschke S, Eckel J. Myokines in insulin resistance and type 2 diabetes. Diabetologia 2014;57:1087–1099. ArticlePubMedPDF
- 29. Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 1998;508(Pt 3):949–953. ArticlePubMedPMC
- 30. Lambernd S, Taube A, Schober A, Platzbecker B, Gorgens SW, Schlich R, et al. Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia 2012;55:1128–1139. ArticlePubMedPDF
- 31. Spiegelman BM. Banting Lecture 2012. Regulation of adipogenesis: toward new therapeutics for metabolic disease. Diabetes 2013;62:1774–1782. ArticlePubMedPMC
- 32. Crujeiras AB, Pardo M, Casanueva FF. Irisin: 'fat' or artefact. Clin Endocrinol (Oxf) 2015;82:467–474. ArticlePubMed
- 33. Choi HY, Kim S, Park JW, Lee NS, Hwang SY, Huh JY, et al. Implication of circulating irisin levels with brown adipose tissue and sarcopenia in humans. J Clin Endocrinol Metab 2014;99:2778–2785. ArticlePubMedPDF
- 34. Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol 2013;9:144–152. ArticlePubMedPDF
- 35. Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes 2002;51:2450–2458. ArticlePubMed
- 36. Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med 2012;18:1279–1285. ArticlePubMedPDF
- 37. Iroz A, Couty JP, Postic C. Hepatokines: unlocking the multi-organ network in metabolic diseases. Diabetologia 2015;58:1699–1703. ArticlePubMedPDF
- 38. Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, et al. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation 2008;118:2555–2562. ArticlePubMed
- 39. Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, et al. Fetuin-A and incident diabetes mellitus in older persons. JAMA 2008;300:182–188. ArticlePubMedPMC
- 40. Jung TW, Youn BS, Choi HY, Lee SY, Hong HC, Yang SJ, et al. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem Pharmacol 2013;86:960–969. ArticlePubMed
- 41. Choi KM, Han KA, Ahn HJ, Lee SY, Hwang SY, Kim BH, et al. The effects of caloric restriction on fetuin-A and cardiovascular risk factors in rats and humans: a randomized controlled trial. Clin Endocrinol (Oxf) 2013;79:356–363. ArticlePubMed
- 42. Bae KH, Kim JG, Park KG. Transcriptional regulation of fibroblast growth factor 21 expression. Endocrinol Metab (Seoul) 2014;29:105–111. ArticlePubMedPMC
- 43. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007;5:426–437. ArticlePubMed
- 44. Yoo HJ, Choi KM. Hepatokines as a link between obesity and cardiovascular diseases. Diabetes Metab J 2015;39:10–15. ArticlePubMedPMC
- 45. Kharitonenkov A, Adams AC. Inventing new medicines: the FGF21 story. Mol Metab 2013;3:221–229. ArticlePubMedPMC
- 46. Gimeno RE, Moller DE. FGF21-based pharmacotherapy: potential utility for metabolic disorders. Trends Endocrinol Metab 2014;25:303–311. ArticlePubMed
- 47. Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010;139:456–463. ArticlePubMedPMC
- 48. Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 2013;18:333–340. ArticlePubMed
- 49. Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab 2010;12:483–495. ArticlePubMed
- 50. Yang SJ, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J Clin Endocrinol Metab 2011;96:E1325–E1329. ArticlePubMed
- 51. Jung TW, Lee SY, Hong HC, Choi HY, Yoo HJ, Baik SH, et al. AMPK activator-mediated inhibition of endoplasmic reticulum stress ameliorates carrageenan-induced insulin resistance through the suppression of selenoprotein P in HepG2 hepatocytes. Mol Cell Endocrinol 2014;382:66–73. ArticlePubMed
- 52. Misu H, Ishikura K, Kurita S, Takeshita Y, Ota T, Saito Y, et al. Inverse correlation between serum levels of selenoprotein P and adiponectin in patients with type 2 diabetes. PLoS One 2012;7:e34952ArticlePubMedPMC
References
Figure & Data
References
Citations

- Cardiometabolic diseases and early cognitive decline: Mitigated by integrated active lifestyle for brain health
Haowei Li, Shige Qi, Shengshu Wang, Shanshan Yang, Shaohua Liu, Shimin Chen, Xuehang Li, Rongrong Li, Junhan Yang, Huaihao Li, Yinghui Bao, Yueting Shi, Zhihui Wang, Miao Liu, Yao He
Journal of Affective Disorders.2024; 350: 155. CrossRef - Current status and future perspectives of FGF21 analogues in clinical trials
Zara Siu Wa Chui, Qing Shen, Aimin Xu
Trends in Endocrinology & Metabolism.2024;[Epub] CrossRef - Organokinler ve Biyokimyasal Etkileri
Ahmet İlhan, Umut Kökbaş
Arşiv Kaynak Tarama Dergisi.2024; 33(1): 71. CrossRef - Pathophysiology of type 2 diabetes and the impact of altered metabolic interorgan crosstalk
Jose Marcos Sanches, Li Na Zhao, Albert Salehi, Claes B. Wollheim, Philipp Kaldis
The FEBS Journal.2023; 290(3): 620. CrossRef - Research Progress on the Mechanism of Obesity-Induced Cardiovascular Disease
文清 刘
Advances in Clinical Medicine.2023; 13(08): 12887. CrossRef - The Pan-liver Network Theory: From Traditional Chinese Medicine to Western Medicine
Yaxing Zhang, Xian-Ming Fang
Chinese Journal of Physiology.2023; 66(6): 401. CrossRef - Higher serum level of CTRP15 in patients with coronary artery disease is associated with disease severity, body mass index and insulin resistance
Abolfazl Shokoohi Nahrkhalaji, Reza Ahmadi, Reza Fadaei, Ghodratollah Panahi, Malihe Razzaghi, Soudabeh Fallah
Archives of Physiology and Biochemistry.2022; 128(1): 276. CrossRef - Non-Alcoholic Steatohepatitis (NASH) and Organokines: What Is Now and What Will Be in the Future
João Paulo Margiotti dos Santos, Mariana Canevari de Maio, Monike Alves Lemes, Lucas Fornari Laurindo, Jesselina Francisco dos Santos Haber, Marcelo Dib Bechara, Pedro Sidnei do Prado, Eduardo Costa Rauen, Fernando Costa, Barbara Cristina de Abreu Pereira
International Journal of Molecular Sciences.2022; 23(1): 498. CrossRef - Hepatic PTEN Signaling Regulates Systemic Metabolic Homeostasis through Hepatokines-Mediated Liver-to-Peripheral Organs Crosstalk
Flavien Berthou, Cyril Sobolewski, Daniel Abegg, Margot Fournier, Christine Maeder, Dobrochna Dolicka, Marta Correia de Sousa, Alexander Adibekian, Michelangelo Foti
International Journal of Molecular Sciences.2022; 23(7): 3959. CrossRef - Non-Alcoholic Fatty Liver Disease and Metabolic Syndrome in Women: Effects of Lifestyle Modifications
Maria Teresa Guagnano, Damiano D'Ardes, Rossi Ilaria, Francesca Santilli, Cosima Schiavone, Marco Bucci, Francesco Cipollone
Journal of Clinical Medicine.2022; 11(10): 2759. CrossRef - Contribution of organokines in the development of NAFLD/NASH associated hepatocellular carcinoma
Meenakshi Vachher, Savita Bansal, Bhupender Kumar, Sandeep Yadav, Taruna Arora, Nalini Moza Wali, Archana Burman
Journal of Cellular Biochemistry.2022; 123(10): 1553. CrossRef - Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise
Giulia Minniti, Letícia Maria Pescinini-Salzedas, Guilherme Almeida dos Santos Minniti, Lucas Fornari Laurindo, Sandra Maria Barbalho, Renata Vargas Sinatora, Lance Alan Sloan, Rafael Santos de Argollo Haber, Adriano Cressoni Araújo, Karina Quesada, Jesse
International Journal of Molecular Sciences.2022; 23(21): 13452. CrossRef - CURRENT CONCEPTS ON LEPTIN-MEDIATED REGULATION OF METABOLISM
R. B. Aliiev
Bulletin of Problems Biology and Medicine.2022; 1(4): 9-1. CrossRef - Inflammatory biomarkers and prediction of insulin resistance in Congolese adults
Reine Freudlendrich Eboka-Loumingou Sakou, Benjamin Longo-Mbenza, Mûnka Nkalla-Lambi, Etienne Mokondjimobe, Henry Germain Monabeka, Donatien Moukassa, Ange Antoine Abena, Mia Pamela Mekieje Tumchou, Venant Tchokonte-Nana
Heliyon.2021; 7(2): e06139. CrossRef - Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions
Ana Rita de Oliveira dos Santos, Bárbara de Oliveira Zanuso, Vitor Fernando Bordin Miola, Sandra Maria Barbalho, Patrícia C. Santos Bueno, Uri Adrian Prync Flato, Claudia Rucco P. Detregiachi, Daniela Vieira Buchaim, Rogério Leone Buchaim, Ricardo José To
International Journal of Molecular Sciences.2021; 22(5): 2639. CrossRef - The Effects of Two Different Intensities of Combined Training on C1q/TNF-Related Protein 3 (CTRP3) and Insulin Resistance in Women with Non-alcoholic Fatty Liver Disease
Somayeh Rajabi, Roya Askari, Amir Hossein Haghighi, Nasrin Razavianzadeh
Hepatitis Monthly.2021;[Epub] CrossRef - Handgrip and sex-specific cardiometabolic risk factors in Hispanic/Latino migrant farmworkers
Anas Raed, Jessica Bilz, Miriam Cortez-Cooper, Lufei Young, Li Chen, Pamela Cromer, Haidong Zhu, Andrew Mazzoli, Samip Parikh, Jigar Bhagatwala, Yutong Dong, Zhuo Sun, Debbie Layman, Yanbin Dong
Scientific Reports.2021;[Epub] CrossRef - Crossing the Antarctica: Exploring the Effects of Appetite-Regulating Hormones and Indicators of Nutrition Status during a 93-Day Solo-Expedition
Bjørn Helge Johnsen, Guttorm Brattebø, Terry M. Phillips, Rune Gjeldnes, Paul T. Bartone, Hans-Olav Neteland Monsen, Julian F. Thayer
Nutrients.2021; 13(6): 1777. CrossRef - Irisin in atherosclerosis
Zhe-Bin Cheng, Liang Huang, Xuan Xiao, Jia-Xiang Sun, Zi-Kai Zou, Jie-Feng Jiang, Cong Lu, Hai-Ya Zhang, Chi Zhang
Clinica Chimica Acta.2021; 522: 158. CrossRef - Pilates and TRX training methods can improve insulin resistance in overweight women by increasing an exercise-hormone, Irisin
Marzyeh Rahimi, Parvaneh Nazarali, Rostam Alizadeh
Journal of Diabetes & Metabolic Disorders.2021; 20(2): 1455. CrossRef - Effects of GLP-1 receptor agonists on myokine levels and pro-inflammatory cytokines in patients with type 2 diabetes mellitus
Valentina Guarnotta, Maria J. Bianco, Enrica Vigneri, Felicia Panto’, Bruna Lo Sasso, Marcello Ciaccio, Giuseppe Pizzolanti, Carla Giordano
Nutrition, Metabolism and Cardiovascular Diseases.2021; 31(11): 3193. CrossRef - Hepatokines and Non-Alcoholic Fatty Liver Disease: Linking Liver Pathophysiology to Metabolism
Tae Hyun Kim, Dong-Gyun Hong, Yoon Mee Yang
Biomedicines.2021; 9(12): 1903. CrossRef - Higher circulating levels of ANGPTL8 are associated with body mass index, triglycerides, and endothelial dysfunction in patients with coronary artery disease
Reza Fadaei, Hossein Shateri, Johanna K. DiStefano, Nariman Moradi, Mohammad Mohammadi, Farzad Emami, Hassan Aghajani, Nasrin Ziamajidi
Molecular and Cellular Biochemistry.2020; 469(1-2): 29. CrossRef - Nonalcoholic fatty liver disease and cardiovascular disease phenotypes
Giandomenico Bisaccia, Fabrizio Ricci, Cesare Mantini, Claudio Tana, Gian Luca Romani, Cosima Schiavone, Sabina Gallina
SAGE Open Medicine.2020; 8: 205031212093380. CrossRef - Dysregulated Autophagy Mediates Sarcopenic Obesity and Its Complications via AMPK and PGC1α Signaling Pathways: Potential Involvement of Gut Dysbiosis as a Pathological Link
Ji Yeon Ryu, Hyung Muk Choi, Hyung-In Yang, Kyoung Soo Kim
International Journal of Molecular Sciences.2020; 21(18): 6887. CrossRef - Fetuin-A as a Potential Biomarker of Metabolic Variability Following 60 Days of Bed Rest
Kiera Ward, Edwin Mulder, Petra Frings-Meuthen, Donal J. O’Gorman, Diane Cooper
Frontiers in Physiology.2020;[Epub] CrossRef - Effect of Moderate Aerobic Exercise on Serum Levels of FGF21 and Fetuin A in Women with Type 2 Diabetes
Exir Vizvari, Parvin farzanegi, Hajar Abbas Zade
Medical Laboratory Journal.2020; 14(6): 17. CrossRef - The implication of hepatokines in metabolic syndrome
Maryam Esfahani, Mostafa Baranchi, Mohammad Taghi Goodarzi
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(4): 2477. CrossRef - The Role of Adipose Tissue and Adipokines in Sepsis: Inflammatory and Metabolic Considerations, and the Obesity Paradox
Irene Karampela, Gerasimos Socrates Christodoulatos, Maria Dalamaga
Current Obesity Reports.2019; 8(4): 434. CrossRef - Sarcopenia and myokines profile as risk factors in cardiovascular diseases?
Mariusz Ciołkiewicz, Anna Kuryliszyn-Moskal, Anna Hryniewicz, Karol Kamiński
Postępy Higieny i Medycyny Doświadczalnej.2019; 73: 550. CrossRef - Pathophysiological Implication of Fetuin-A Glycoprotein in the Development of Metabolic Disorders: A Concise Review
Lynda Bourebaba, Krzysztof Marycz
Journal of Clinical Medicine.2019; 8(12): 2033. CrossRef - Loss of Glycine N-Methyltransferase Associates with Angiopoietin-Like Protein 8 Expression in High Fat-Diet-Fed Mice
Jian-Wei Huang, Chao-Ju Chen, Chia-Hung Yen, Yi-Ming Arthur Chen, Yu-Peng Liu
International Journal of Molecular Sciences.2019; 20(17): 4223. CrossRef - Recent advances in biosensor technology in assessment of early diabetes biomarkers
Armin Salek-Maghsoudi, Faezeh Vakhshiteh, Raheleh Torabi, Shokoufeh Hassani, Mohammad Reza Ganjali, Parviz Norouzi, Morteza Hosseini, Mohammad Abdollahi
Biosensors and Bioelectronics.2018; 99: 122. CrossRef - Insulin-stimulated glucose uptake in skeletal muscle, adipose tissue and liver: a positron emission tomography study
Miikka-Juhani Honka, Aino Latva-Rasku, Marco Bucci, Kirsi A Virtanen, Jarna C Hannukainen, Kari K Kalliokoski, Pirjo Nuutila
European Journal of Endocrinology.2018; 178(5): 523. CrossRef - Magnetic multiwalled carbon nanotubes as nanocarrier tags for sensitive determination of fetuin in saliva
Esther Sánchez-Tirado, Araceli González-Cortés, Paloma Yáñez-Sedeño, José M. Pingarrón
Biosensors and Bioelectronics.2018; 113: 88. CrossRef - Decreased muscle mass in Korean subjects with intracranial arterial stenosis: The Kangbuk Samsung Health Study
Ho-Jung Jung, Hwanseok Jung, Taeyoung Lee, Jongho Kim, Jongsin Park, Hacsoo Kim, Junghwan Cho, Won-Young Lee, Sung-Woo Park, Eun-Jung Rhee, Hyung-Geun Oh
Atherosclerosis.2017; 256: 89. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: findings from the KoGES
Jang Won Son, Seong Su Lee, Sung Rae Kim, Soon Jib Yoo, Bong Yun Cha, Ho Young Son, Nam H. Cho
Diabetologia.2017; 60(5): 865. CrossRef - Effects of chlorogenic acid on intracellular calcium regulation in lysophosphatidylcholine-treated endothelial cells
Hye-Jin Jung, Seung-Soon Im, Dae-Kyu Song, Jae-Hoon Bae
BMB Reports.2017; 50(6): 323. CrossRef - Irisin: A Potential Link between Physical Exercise and Metabolism—An Observational Study in Differently Trained Subjects, from Elite Athletes to Sedentary People
Stefano Benedini, Elena Dozio, Pietro Luigi Invernizzi, Elena Vianello, Giuseppe Banfi, Ileana Terruzzi, Livio Luzi, Massimiliano Marco Corsi Romanelli
Journal of Diabetes Research.2017; 2017: 1. CrossRef - Serum Vaspin Concentration in Elderly Type 2 Diabetes Mellitus Patients with Differing Body Mass Index: A Cross-Sectional Study
Wei Yang, Yun Li, Tian Tian, Li Wang
BioMed Research International.2017; 2017: 1. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite