Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(2); 2022 > Article
-
Review ArticleThyroid Thyroid Function across the Lifespan: Do Age-Related Changes Matter?
 Keypoint
Keypoint
Circulating concentrations of thyrotropin (TSH) and thyroxine (T4) are tightly regulated. Each individual has a genetically determined set point for TSH and free T4 that is also subject to environmental and epigenetic influences. In later life, TSH increases with age in healthy older adults without an accompanying fall in free T4, indicating an alteration in the TSH set point. There is a strong case for implementing age-related reference ranges for TSH in adults to prevent an inappropriate diagnosis of subclinical hypothyroidism in older people and to discourage unnecessary levothyroxine prescribing. -
John P. Walsh1,2

-
Endocrinology and Metabolism 2022;37(2):208-219.
DOI: https://doi.org/10.3803/EnM.2022.1463
Published online: April 14, 2022
1Department of Endocrinology & Diabetes, Sir Charles Gairdner Hospital, Nedlands, Australia
2Medical School, University of Western Australia, Crawley, Australia
- Corresponding author: John P. Walsh. Department of Endocrinology & Diabetes, Sir Charles Gairdner Hospital, Hospital Avenue, Nedlands, Western Australia 6009, Australia Tel: +61-864572466, Fax: +61-864573221, E-mail: john.walsh@health.wa.gov.au
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Circulating concentrations of thyrotropin (TSH) and thyroxine (T4) are tightly regulated. Each individual has setpoints for TSH and free T4 which are genetically determined, and subject to environmental and epigenetic influence. Pituitary-thyroid axis setpoints are probably established in utero, with maturation of thyroid function continuing until late gestation. From neonatal life (characterized by a surge of TSH and T4 secretion) through childhood and adolescence (when free triiodothyronine levels are higher than in adults), thyroid function tests display complex, dynamic patterns which are sexually dimorphic. In later life, TSH increases with age in healthy older adults without an accompanying fall in free T4, indicating alteration in TSH setpoint. In view of this, and evidence that mild subclinical hypothyroidism in older people has no health impact, a strong case can be made for implementation of age-related TSH reference ranges in adults, as is routine in children.
- Thyroid hormones have profound and widespread physiological effects, including regulation of metabolism and thermogenesis. Thyroid hormones also play key roles in growth and development which are phylogenetically diverse [1]. For example, over 100 years ago, it was shown that extracts of mammalian thyroid induce metamorphosis in tadpoles [2], whereas thyroidectomy prevents metamorphosis and arrests development [3]. Not long afterward, thyroidectomy was shown to disrupt the seasonality of breeding in ducks, songbirds, and sheep, and it is now known that thyroid hormone action on the mediobasal hypothalamus is essential to seasonal reproductive cycling across vertebrate species [4,5]. Key roles of thyroid hormones in human growth and development are also well established, as demonstrated by disorders such as congenital hypothyroidism and thyroid hormone resistance alpha [6,7]. The purpose of this review is to provide an overview of pituitary-thyroid axis function across the human lifespan.
INTRODUCTION
- In a classic study by Andersen et al. [8], it was shown that in healthy individuals studied repeatedly over a year, circulating levels of thyrotropin (TSH) and thyroxine (T4) are tightly regulated, with much less variation observed within a given individual across time than between individuals. The physiological ranges for each individual (reflecting biological variation) are therefore narrower than the population-derived laboratory reference ranges. This gives rise to the key concept that each individual has set points (individual means) for TSH and T4, and what is normal for one individual may not be normal for another, even within conventional definitions of euthyroidism [9]. Notably, circulating TSH exists in several different isoforms with varying degrees of glycosylation, sialylation, and sulfonation which affect tissue availability and bioactivity [10,11]; this is not reflected in immunoreactive TSH concentrations determined by routine laboratory assays.
- Setpoints for hypothalamo-pituitary-thyroid (HPT) axis function are probably established in utero. Evidence for this comes from individuals with congenital hypothyroidism in whom, despite early detection and treatment of hypothyroidism, the relationship between TSH and free T4 may be altered through childhood and adult life. In one study, at any given TSH concentration, children with congenital hypothyroidism had higher free T4 concentrations than children with autoimmune hypothyroidism [12]. Similarly, when individuals with congenital hypothyroidism were studied as adults, higher levothyroxine dosage and higher free T4 concentrations were required to achieve the same TSH concentration compared with patients with hypothyroidism from total thyroidectomy [13].
- Pituitary-thyroid axis setpoints are to a large extent heritable traits. Estimates of heritability vary [14-18], but are up to 60% to 70% for each of TSH, free T4, and free triiodothyronine (T3) (Table 1). In recent years, there have been substantial advances in understanding the genetic architecture of pituitary-thyroid axis function [19]. This started with candidate gene studies, then genome wide association studies, initially using gene chips with relatively sparse coverage of the human genome in small populations, and more recently in large populations with more densely spaced genetic markers and high levels of imputation. This approach has been highly successful in identifying common genetic variants associated with thyroid function. In the most recent meta-analysis of 32 cohorts with a total sample size of more than 70,000 participants, 42 independent genetic loci were found to be associated with TSH, and 21% with free T4 [20]. Even with this large-scale effort, however, only 33% of genetic variance in TSH and 21% of variance in free T4 were accounted for. The “missing heritability” of thyroid function probably arises from multiple (as yet unidentified) genetic variants, each with small effects, requiring even larger studies for detection [21]. Rare variants (defined as a minor allele frequency <1%) are also likely to be important; although individually rare, these are collectively quite frequent and may have greater individual effect sizes than do common variants. In a whole genome sequencing study, several rare variants were identified as associated with thyroid function [22], and larger studies are required to build on this.
- Epigenetic mechanisms such as DNA methylation, phosphorylation, acetylation and histone modification regulate gene expression without altering the DNA nucleotide sequence. This provides a potential link between environmental influences, gene expression and thyroid function [23]. Although non-permanent, epigenetic modifications can be transmitted to subsequent generations, and may account for some of the missing heritability of thyroid function [24]. Our group recently published the first epigenome-wide association study of thyroid function in healthy individuals, using methylation data from leukocyte DNA from two cohorts of adolescent Australians [25]. We identified two differentially methylated positions (DMPs) associated with TSH, none with free T4 and six with free T3, including DMPs in KLF9 and DOT1L, both genes which are known to be induced by T3. Further research is required to explore the relevance of these loci to pituitary-thyroid axis physiology and thyroid hormone action. DNA methylation shows marked changes across the lifespan [23,24], and is likely to be relevant to age-related changes in HPT axis function.
PITUITARY-THYROID AXIS SETPOINTS
- There are numerous environmental influences on HPT axis function. At the population level, the most important is iodine status [26]. The clinical impact of severe iodine deficiency is well-known, but even small differences in iodine status can affect the population distribution of TSH and alter the relationship between TSH and age [27-31]. Serum TSH, and to a lesser extent free T4 and free T3, is also affected by photoperiod and temperature, exhibiting circadian, seasonal and circannual variation [32-37]. The magnitude of these physiological effects can be clinically relevant: for example, the increase in TSH during winter can cause seasonal variation in the diagnosis of subclinical hypothyroidism [38]. Tobacco smoking affects thyroid function [39], such that current smokers have lower mean TSH concentrations than people who have never smoked. After smoking cessation, TSH levels gradually normalize over a prolonged period of 10 years or more [40].
ENVIRONMENTAL INFLUENCES
- Thyroid embryogenesis is largely complete by 7 weeks gestation, but terminal differentiation of the gland, characterized by follicle formation and thyroid hormone synthesis, does not occur until 10 to 12 weeks [41-44]. TSH, T4, and T3 are detectable in fetal circulation from 11 to 12 weeks onwards, but physiologically relevant thyroid hormone secretion does not occur until the second trimester. It is often stated that the fetal HPT axis is functionally mature by 18 to 20 weeks gestation [45,46], but in fact there is good evidence that maturation continues through the third trimester. These data come from studies utilizing cordocentesis for fetal blood sampling in utero or cord blood samples from fetuses born prematurely or undergoing termination of pregnancy [41,47-49]. The data are not completely consistent across studies, but it appears that fetal TSH levels are approximately 4 mU/L at 12 weeks, increase to 7 mU/L at 28 weeks, then remain stable until term. During the third trimester, fetal TSH is poorly sialylated and more bioactive than in older children and adults [50]; whether this is true earlier in gestation is not known. Serum total T4 concentrations in the fetus are low at 12 weeks gestation (~20 nmol/L), increase to ~70 nmol/L by 28 weeks then increase further to ~120 nmol/L at term, similar to levels in older children. Thyroxine-binding globulin (TBG) levels are low at 12 weeks, then increase progressively until term. Free T4 concentrations are also low (~2 pmol/L) at 12 weeks, increase to 15 to 20 pmol/L at 28 weeks, then plateau until term, influenced by the increase in TBG. Fetal total T3 levels are barely detectable at 12 weeks gestation, increase to ~0.4 nmol/L by 28 weeks and increase further to ~0.9 nmol/L at term, still lower than in older children.
- Prior to maturation of the fetal thyroid, transplacental passage of maternal T4 occurs and plays an important role in fetal brain development. Transplacental transfer of T4 continues until fetal free T4 concentrations approximate maternal free T4 levels at ~28 weeks gestation [51]; in fetuses with congenital hypothyroidism, transplacental passage of T4 continues until term [52].
THYROID FUNCTION IN UTERO
- Immediately after birth, there is a surge in TSH secretion which peaks at around 30 minutes postnatally, with levels as high as 60 to 80 mU/L. This is followed by an increase in T4 which in turn peaks during the first day of life, when free T4 levels which can be twice as high as in older children or adults [49,53]. TSH and free T4 levels fall during the first month of life, but can still be above levels seen in adults [49,54,55]. Circulating T3 concentrations are low at birth, increase during the first week of life, and remain elevated across the first neonatal month. Studies of babies who were warmed or cooled in the first few hours and days of life suggest that high TSH levels during the first month of life are a response to the temperature drop between the intrauterine and extrauterine environments, although this may not be true of the early TSH surge immediately after birth, which appears to be temperature-independent [53]. In premature infants, the neonatal TSH surge in the first day of life is attenuated compared with term infants; the T4 surge is also attenuated in infants born at 28 to 34 weeks gestation and is absent in severely premature babies born at 23 to 27 weeks [49,56]. This, along with other factors, predisposes to hypothyroxinemia in premature infants [44], and provides further evidence that the fetal pituitary-thyroid axis is not fully mature until late gestation.
- After the first month of life, TSH levels remain somewhat higher than in adults, gradually falling and plateauing at 6 to 9 months of age, then remaining largely stable through the rest of childhood, accompanied by a gradual fall in free T4 concentrations across this period [54]. The predominant TSH isoforms in children up to 18 months of age have low sialylation and N-glycosylation, resulting in high bioactivity, after which TSH glycobiology becomes similar to that seen in adults [57]. Circulating T3 levels peak at 4 to 5 months of age, then gradually decline. At all ages, mean TSH levels are slightly higher in boys than girls.
- Reference ranges for TSH, free T4, and free T3 in children are typically wider than in adults, but are not well-standardized or harmonized. In a recent review, Onsesveren et al. [58] reported considerable variation: for example, for children aged 7 days to 3 months, reference interval lower limits for TSH ranged from 0.16 to 1.80 mU/L and upper limits from 4.38 to 12.56 mU/L; for 5 to 10 years of age the lower limits were 0.48 to 1.30 mU/L and upper limits 3.36 to 5.66 mU/L. Likely contributors to this variation include differences between studies in assay methods and statistical analysis, as well as iodine status, ethnicity, and anthropometric factors.
- Children who develop hypothyroidism require higher doses of levothyroxine per kilogram (kg) of body weight than do adults, approximating 10 to 15 µg/kg/day in neonates, 4 to 6 µg/kg/day from age 1 to 5 years, and 2 to 4 µg/kg/day in older children and adolescents, whereas adults typically require 1.6 to 2.0 µg/kg/day [59]. This indicates the high level of metabolic and secretory activity of the thyroid during childhood.
EARLY LIFE AND CHILDHOOD
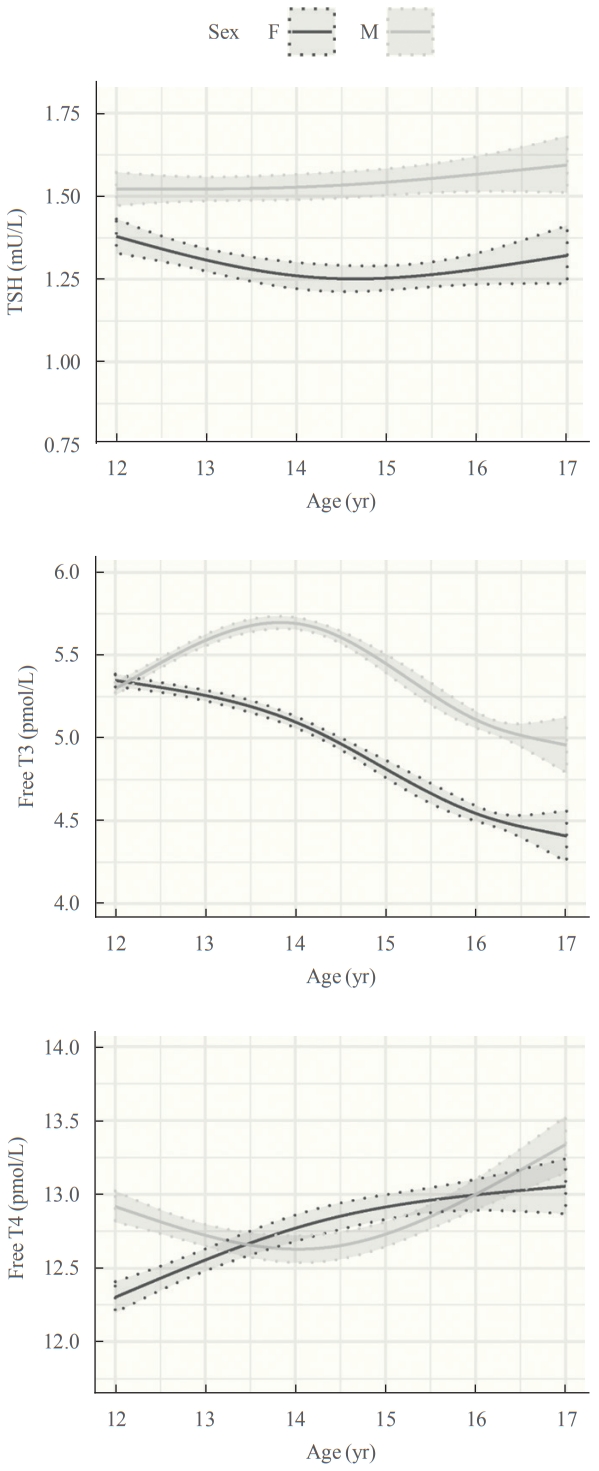
- Adolescence is an important time of growth and development in which the thyroid plays a key role. We recently published the first detailed longitudinal analysis of thyroid function in an adolescent cohort [60], finding complex, sexually dimorphic patterns of thyroid function tests (Fig. 1). TSH was consistently higher in males than females with stable values from age 12 to 14 years, then an increase from age 14 to 16 in both sexes. In girls, free T4 increased from age 12 to 14 while remaining unchanged in boys, then increased from age 14 to 16 in both sexes. Free T3 was higher at all time points in boys than girls, and trajectories showed a marked sex difference. In girls, there was minimal change in free T3 from age 12 to 14, then a sharp decline to age 16, whereas in boys free T3 increased from age 12 to 14 then declined by age 16. These data are broadly consistent with limited data from other longitudinal studies [61,62] and cross-sectional studies [63]. The physiological basis for these changes is not fully understood. The sex differences (particularly for free T3) are partly explained by the earlier age of puberty in girls than boys, and the reciprocal changes in free T4 and free T3 concentrations suggest altered activity of one or more of the iodothyronine deiodinases, probably as a result of increased secretion of sex hormones, growth hormone/insulin growth factor 1 and other factors during puberty.
- These results are clinically relevant, because reference intervals for thyroid function tests clearly differ between adolescents and adults. For TSH and free T4, the differences were relatively minor, reflecting narrower distributions in teenagers than adults. For free T3, however, there is an upward shift in concentrations in adolescents than adults, most evident in 12-year-old girls and 14-year-old boys. In these age groups, use of adult reference ranges would misclassify 35% of girls and 58% of boys as having abnormal free T3 levels.
ADOLESCENCE
- In healthy, older adults without thyroid disease, it was previously thought there was an age-related reduction in TSH levels [64,65], but this was based largely on studies from iodine-deficient populations after exclusion of individuals with subclinical hypothyroidism, which biased the results. In studies of iodinesufficient populations, including the National Health and Nutrition Examination Survey in the United States (NHANES III), it was shown convincingly that in healthy individuals with no evidence of thyroid disease, there is in fact a progressive increase in TSH concentrations with increasing age which is apparent from the third decade of life [66,67]. A limitation of NHANES III was the cross-sectional design, but an age-related increase in TSH secretion was subsequently confirmed in two longitudinal studies. In a reference group of Busselton Health Study participants with no evidence of thyroid disease studied on two occasions, 13 years apart (mean age at baseline 45.5 years), there was a significant increase in mean TSH concentrations with no change in mean free T4 levels [68]. In a second longitudinal study of older people (mean age 72 at baseline), also studied on two occasions, 13 years apart, there was an identical increase in mean TSH in a disease-free subcohort, accompanied by a small increase in mean free T4 [69]. These results were not replicated in a third study (mean age at baseline 65 years), in which mean TSH did not change significantly over time [70], probably because of the shorter duration of follow-up (6.5 years).
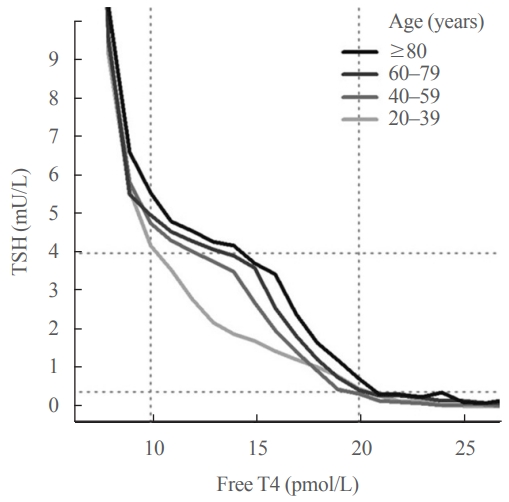
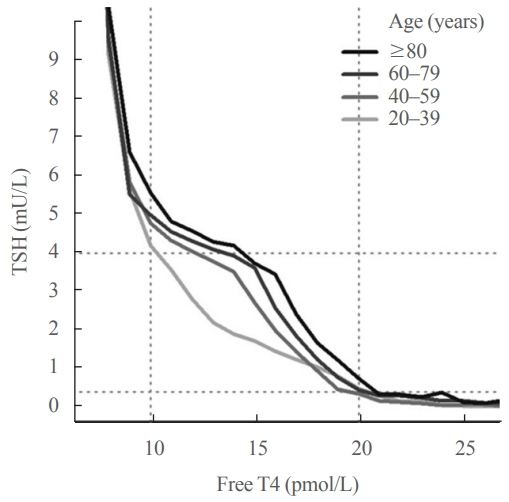
- Further insight into the age-related increase in TSH comes from examining the relationship between TSH and free T4. A key feature of the TSH-free T4 relationship is that small changes in circulating free T4 concentrations result in relatively large changes in TSH. This accounts for the high community prevalence of subclinical thyroid disease, since TSH is frequently out of range accompanied by normal free T4 rather than the other way around, and underpins the “TSH-first” approach in screening for thyroid disease [71,72]. It was previously thought that there was a simple inverse log-linear relationship between TSH and free T4 [73-75], but it is now known that the relationship between log TSH and free T4 is complex and non-linear [76,77]. In an analysis of over 120,000 individuals from a laboratory database [72], we reported that the TSH-free T4 relationship differed across age groups, such that at any given free T4 concentration within the reference range, older people had higher TSH concentrations than younger people (Fig. 2), suggesting an age-related alteration in TSH setpoint.
- The mechanism for the age-related in TSH increase with aging is uncertain, and there are several possibilities. Firstly, it is possible (but largely untested) that TSH isoforms change with aging, resulting in reduced TSH bioactivity in older adults [11,78]. Secondly, there could be attenuation of the negative feedback effect of free T4 on TSH secretion, for example by reduced type 2 iodothyronine deiodinase activity in the pituitary. Thirdly, there could be a reduction in thyrocyte responsiveness to TSH, requiring higher TSH concentrations to maintain the same free T4 level. More research is required to address this question.
THYROID FUNCTION IN ADULT LIFE
- The age-related increase in TSH, occurring without an accompanying fall in free T4 has important clinical implications. Firstly, it raises the question of whether age-related reference ranges for TSH should be applied to older adults. Following the publication of NHANES III data discussed above, this was advocated [79], but has not been widely adopted. One reason for this may be that in the early 2000s, there was robust debate about the TSH reference range in general, and in particular whether the TSH reference upper limit should be reduced from approximately 4–4.5 to 2.5 or 3.0 mU/L [80,81]. This debate is now largely settled, in favor of a TSH upper limit remaining at 4.0 to 4.5 mU/L [29,82], but may have overshadowed the important question of age-related ranges.
- Table 2 shows examples of age-related reference ranges from selected studies in the literature, using young adults aged under 30 years as the comparator group [31,68,83-88]. A consistent feature is that the upper limit for TSH (defined as the 97.5th centile) is substantially higher in older age groups, being 0.5 to 2.7 mU/L greater than for young adults, whereas the lower limit of the reference range is not affected by age. The absolute values for TSH reference range limits differ between studies, reflecting methodological differences (e.g., definition of diseasefree groups, availability of thyroid antibody measurements) as well as preanalytic and analytic factors. These include environmental factors (especially iodine status and smoking, and to a lesser extent sex, season and temperature), ethnicity (which is known to impact TSH reference ranges [67,79,83]) and analytic factors, as TSH assays are poorly harmonized, with inter-assay differences of up to 1 mU/L at high-normal TSH concentrations [84,89]. Ideally, therefore, TSH reference ranges should be generated by laboratories from local population-based data where possible.
- Application of age-related reference ranges for TSH would reduce the number of older people regarded as abnormal on the basis of a mildly elevated TSH. This would be desirable, as the natural history of TSH elevations in older people appears favorable. TSH can increase as a result of nonthyroidal systemic illness, transient thyroiditis or simply biological variation, and often returns to the reference range on repeat testing [90]. For that reason, it is recommended that subclinical hypothyroidism be confirmed by repeat testing before levothyroxine treatment is started, but in clinical practice, patients are frequently started on treatment after a single measurement of TSH [91], and many continue treatment lifelong which may be unnecessary [92] and, if not adequately monitored, potentially harmful [93,94].
- Even when confirmed on repeat testing, mild subclinical hypothyroidism in older people appears to have no health impact. A recent combined analysis of data from participants aged ≥80 years or more in two randomized controlled trials reported no benefit of levothyroxine treatment for subclinical hypothyroidism [95]. This is consistent with the full results of one of the included trials, which found no symptomatic treatment benefit in people aged ≥65 years or more [96]. Observational studies suggest that mild TSH elevation in older people does not predict adverse health outcomes. In fact, higher TSH is associated with greater life expectancy, including extreme longevity [69,78,97-101]. Although subclinical hypothyroidism is a risk factor for cardiovascular disease, a meta-analysis of individual participant data from 11 cohort studies found that this only applied to TSH levels ≥7.0 mU/L, with no increased risk associated with TSH 4.5 to 7.0 mU/L [102]. More recently, a study level meta-analysis of 35 cohort studies found that subclinical hypothyroidism was a predictor of mortality and cardiovascular outcomes only in cohorts which were younger (median age <65 years) and/or had high predicted cardiovascular risk at baseline [103]. In an informative community-based cohort study, stratified analysis showed that TSH in the highest quartile of subclinical hypothyroidism (TSH >6.57 mU/L) was a predictor of cardiovascular mortality and events (whereas TSH 4.0 to 6.57 mU/L was not), and that within this subgroup, participants aged ≥65 years were not at increased risk unless they had pre-existing cardiovascular risk factors [104]. Finally, in a large observational study from the United Kingdom, levothyroxine treatment of people with TSH between 5 and 10 mU/L was associated with reduced cardiovascular risk and overall mortality in patients aged 40 to 70 years (compared with people left untreated), but not in those aged over 70 years [105].
- These data provide support a hypothesis advanced some years ago that subclinical hypothyroidism is a cardiovascular risk factor from middle age up to 65 or 70 years of age, but not in older people, whereas in the very elderly (over 85 years) it may be associated with prolonged survival [106,107]. In light of this, several clinical guidelines now recommend against routine levothyroxine treatment in older people with mild subclinical hypothyroidism [108-110]. In a recent editorial, Cappola [111] suggested that the upper limit of the TSH reference range be extended to 7 mU/L for people over the age of 80. This would probably reduce unnecessary levothyroxine prescribing for older people, but this dichotomous approach is less physiological and intellectually less appealing than applying graduated or continuous age-related reference ranges to all adults.
IMPLICATIONS OF AGE-RELATED TSH INCREASE
- This review highlights the considerable changes which occur physiologically in HPT axis function across the lifespan. Setpoints for thyroid function are to a large degree heritable and probably established in utero, but much of the underlying genetic architecture remains undescribed, and an understanding of epigenetic influences is only beginning to emerge. There are complex, dynamic changes in thyroid function tests across childhood and adolescence and also in adulthood, such that in older people TSH increases with aging without an accompanying fall in free T4. Age-related reference ranges for thyroid function tests should be routinely used for children and adolescents. There is a strong case for implementing age-related reference ranges for TSH in adults to prevent inappropriate diagnosis of subclinical hypothyroidism in older people and to discourage unnecessary levothyroxine prescribing.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
| Variable | Hansen et al. (2004) [14] | Samollow et al. (2004) [15] | Panicker et al. (2008) [16] | Alul et al. (2013) [17] | Nolan et al. (2021) [18] |
|---|---|---|---|---|---|
| TSH | 0.64 | 0.32 | 0.65 | 0.58 | 0.71 |
| Free T4 | 0.65 | 0.37 | 0.39 | - | 0.67 |
| Free T3 | 0.64 | 0.67 | 0.23 | - | 0.60 |
| Study | Location | Number | Assay |
TSH, mU/L |
|||
|---|---|---|---|---|---|---|---|
| Age 20–30 years | Age 60–70 years | Age 70–80 years | Age 80–90 years | ||||
| Boucai et al. (2011) [83] | USA | 13,296 | Nichols | 0.40–3.60 | 0.46–4.70 | 0.47–5.60 | 0.44–6.30 |
| Kahapola-Arachchige et al. (2012) [84] | Australia | 148,938 | Siemens Centaur | 0.49–3.67 | 0.52–4.32 | 0.48–4.52 | 0.47–4.9 |
| Bremner et al. (2012) [68] | Australia | 1,751 | Immulite | 0.51–3.54 | 3.48–4.70 | 0.52–5.28a | - |
| Vadiveloo et al. (2013) [85] | Scotland | 153,127 | Roche | 0.52–4.15 | 0.48–4.59 | 0.40–4.96 | 0.36–5.49 |
| Farrell et al. (2017) [86] | Australia | 604,194 | Siemens Centaur | 0.53–3.86 | 0.52–4.43 | 0.55–4.66 | 0.52–4.89 |
| Park et al. (2018) [31] | Korea | 5,987 | Roche | 0.67–6.05 | 0.56–7.77 | 0.42–6.68a | - |
| Mokhtar (2020) [87]b | Algeria | 8,838 | Abbott Architect | 0.46–3.90 | 0.42–5.10 | 0.36–5.30a | - |
| Raverot et al. (2020) [88] | France | 156,025 | Abbott Architect | 0.31–4.37 | 0.24–4.72 | 0.24–4.88 | 0.25–4.92 |
Data are from selected studies of populations thought to be free of thyroid disease (disease-free or reference populations) [31,68,83-88]. In some studies, sex-specific reference intervals were provided for men and women which have been combined to a single range for the purposes of this table. Young adults are shown as age 20 to 30 years, but in some studies data are for age 18 to 30 years.
a Data shown are for age >70 years;
b Data from Bhattacharya analysis (one of several analyses in the paper).
- 1. Feingold KR, Anawalt B, Boyce A. South Dartmouth: MDText.com; 2000. Chapter, Ontogeny, anatomy, metabolism and physiology of the thyroid. 2011 [cited 2022 Apr 5]. Available from: www.endotext.org.
- 2. Gudernatsch JF. Feeding experiments on tadpoles. 1. The influence of specific organs given as food on growth and differentiation: a contribution to the knowledge of organs with internal secretion. Arch Entwicklngsrnechn Organ 1912;35:457–84.
- 3. Allen BM. The results of thyroid removal in the larvae of Rana pipiens. J Exp Zool 1918;24:499–519.Article
- 4. Sharp PJ. Photoperiodic regulation of seasonal breeding in birds. Ann N Y Acad Sci 2005;1040:189–99.ArticlePubMed
- 5. Dardente H, Hazlerigg DG, Ebling FJ. Thyroid hormone and seasonal rhythmicity. Front Endocrinol (Lausanne) 2014;5:19.ArticlePubMedPMC
- 6. Peters C, van Trotsenburg AS, Schoenmakers N. Diagnosis of endocrine disease: congenital hypothyroidism: update and perspectives. Eur J Endocrinol 2018;179:R297–317.ArticlePubMed
- 7. Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, et al. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med 2012;366:243–9.ArticlePubMed
- 8. Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 2002;87:1068–72.ArticlePubMed
- 9. Dayan CM, Saravanan P, Bayly G. Whose normal thyroid function is better: yours or mine? Lancet 2002;360:353.ArticlePubMed
- 10. Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev 2002;82:473–502.ArticlePubMed
- 11. Estrada JM, Soldin D, Buckey TM, Burman KD, Soldin OP. Thyrotropin isoforms: implications for thyrotropin analysis and clinical practice. Thyroid 2014;24:411–23.ArticlePubMedPMC
- 12. Kempers MJ, van Trotsenburg AS, van Tijn DA, Bakker E, Wiedijk BM, Endert E, et al. Disturbance of the fetal thyroid hormone state has long-term consequences for treatment of thyroidal and central congenital hypothyroidism. J Clin Endocrinol Metab 2005;90:4094–100.ArticlePubMed
- 13. Bagattini B, Cosmo CD, Montanelli L, Piaggi P, Ciampi M, Agretti P, et al. The different requirement of L-T4 therapy in congenital athyreosis compared with adult-acquired hypothyroidism suggests a persisting thyroid hormone resistance at the hypothalamic-pituitary level. Eur J Endocrinol 2014;171:615–21.ArticlePubMed
- 14. Hansen PS, Brix TH, Sorensen TI, Kyvik KO, Hegedus L. Major genetic influence on the regulation of the pituitarythyroid axis: a study of healthy Danish twins. J Clin Endocrinol Metab 2004;89:1181–7.ArticlePubMed
- 15. Samollow PB, Perez G, Kammerer CM, Finegold D, Zwartjes PW, Havill LM, et al. Genetic and environmental influences on thyroid hormone variation in Mexican Americans. J Clin Endocrinol Metab 2004;89:3276–84.ArticlePubMed
- 16. Panicker V, Wilson SG, Spector TD, Brown SJ, Falchi M, Richards JB, et al. Heritability of serum TSH, free T4 and free T3 concentrations: a study of a large UK twin cohort. Clin Endocrinol (Oxf) 2008;68:652–9.ArticlePubMed
- 17. Alul FY, Cook DE, Shchelochkov OA, Fleener LG, Berberich SL, Murray JC, et al. The heritability of metabolic profiles in newborn twins. Heredity (Edinb) 2013;110:253–8.ArticlePubMed
- 18. Nolan J, Campbell PJ, Brown SJ, Zhu G, Gordon S, Lim EM, et al. Genome-wide analysis of thyroid function in Australian adolescents highlights SERPINA7 and NCOA3. Eur J Endocrinol 2021;185:743–53.ArticlePubMed
- 19. Kus A, Chaker L, Teumer A, Peeters RP, Medici M. The genetic basis of thyroid function: novel findings and new approaches. J Clin Endocrinol Metab 2020;105:dgz225.PubMed
- 20. Teumer A, Chaker L, Groeneweg S, Li Y, Di Munno C, Barbieri C, et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun 2018;9:4455.PubMedPMC
- 21. Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature 2009;461:747–53.ArticlePubMedPMC
- 22. Taylor PN, Porcu E, Chew S, Campbell PJ, Traglia M, Brown SJ, et al. Whole-genome sequence-based analysis of thyroid function. Nat Commun 2015;6:5681.ArticlePubMed
- 23. Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet 2012;13:97–109.ArticlePubMed
- 24. Trerotola M, Relli V, Simeone P, Alberti S. Epigenetic inheritance and the missing heritability. Hum Genomics 2015;9:17.ArticlePubMedPMC
- 25. Lafontaine N, Campbell PJ, Castillo-Fernandez JE, Mullin S, Lim EM, Kendrew P, et al. Epigenome-wide association study of thyroid function traits identifies novel associations of fT3 with KLF9 and DOT1L. J Clin Endocrinol Metab 2021;106:e2191–202.ArticlePubMedPMCPDF
- 26. Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 2015;3:286–95.ArticlePubMed
- 27. Knudsen N, Bulow I, Jorgensen T, Laurberg P, Ovesen L, Perrild H. Comparative study of thyroid function and types of thyroid dysfunction in two areas in Denmark with slightly different iodine status. Eur J Endocrinol 2000;143:485–91.ArticlePubMed
- 28. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006;354:2783–93.ArticlePubMed
- 29. Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011;7:232–9.ArticlePubMed
- 30. van de Ven AC, Netea-Maier RT, Smit JW, Kusters R, van der Stappen JW, Pronk-Admiraal CJ, et al. Thyrotropin versus age relation as an indicator of historical iodine intake. Thyroid 2015;25:629–34.ArticlePubMed
- 31. Park SY, Kim HI, Oh HK, Kim TH, Jang HW, Chung JH, et al. Age- and gender-specific reference intervals of TSH and free T4 in an iodine-replete area: data from Korean National Health and Nutrition Examination Survey IV (2013-2015). PLoS One 2018;13:e0190738.ArticlePubMedPMC
- 32. Fisher DA. Physiological variations in thyroid hormones: physiological and pathophysiological considerations. Clin Chem 1996;42:135–9.ArticlePubMed
- 33. Ehrenkranz J, Bach PR, Snow GL, Schneider A, Lee JL, Ilstrup S, et al. Circadian and circannual rhythms in thyroid hormones: determining the TSH and free T4 reference intervals based upon time of day, age, and sex. Thyroid 2015;25:954–61.ArticlePubMed
- 34. Wang D, Cheng X, Yu S, Qiu L, Lian X, Guo X, et al. Data mining: seasonal and temperature fluctuations in thyroidstimulating hormone. Clin Biochem 2018;60:59–63.ArticlePubMed
- 35. Yoshihara A, Noh JY, Watanabe N, Iwaku K, Kunii Y, Ohye H, et al. Seasonal changes in serum thyrotropin concentrations observed from big data obtained during six consecutive years from 2010 to 2015 at a single hospital in Japan. Thyroid 2018;28:429–36.ArticlePubMed
- 36. Ikegami K, Refetoff S, Van Cauter E, Yoshimura T. Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol 2019;15:590–600.ArticlePubMedPMC
- 37. Kuzmenko NV, Tsyrlin VA, Pliss MG, Galagudza MM. Seasonal variations in levels of human thyroid-stimulating hormone and thyroid hormones: a meta-analysis. Chronobiol Int 2021;38:301–17.ArticlePubMed
- 38. Kim TH, Kim KW, Ahn HY, Choi HS, Won H, Choi Y, et al. Effect of seasonal changes on the transition between subclinical hypothyroid and euthyroid status. J Clin Endocrinol Metab 2013;98:3420–9.ArticlePubMed
- 39. Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf) 2013;79:145–51.ArticlePubMed
- 40. Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Arch Intern Med 2007;167:1428–32.ArticlePubMed
- 41. Thorpe-Beeston JG, Nicolaides KH, McGregor AM. Fetal thyroid function. Thyroid 1992;2:207–17.ArticlePubMed
- 42. Szinnai G, Lacroix L, Carre A, Guimiot F, Talbot M, Martinovic J, et al. Sodium/iodide symporter (NIS) gene expression is the limiting step for the onset of thyroid function in the human fetus. J Clin Endocrinol Metab 2007;92:70–6.ArticlePubMed
- 43. Nilsson M, Fagman H. Development of the thyroid gland. Development 2017;144:2123–40.ArticlePubMed
- 44. LaFranchi SH. Thyroid function in preterm/low birth weight infants: impact on diagnosis and management of thyroid dysfunction. Front Endocrinol (Lausanne) 2021;12:666207.ArticlePubMedPMC
- 45. Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol 2017;13:610–22.ArticlePubMed
- 46. Feingold KR, Anawalt B, Boyce A. Endotext. South Dartmouth: MDText.com; 2000. Chapter, Disorders of the thyroid gland in infancy, childhood and adolescence. 2011 [cited 2022 Apr 5]. Available from: www.endotext.org.
- 47. Thorpe-Beeston JG, Nicolaides KH, Felton CV, Butler J, McGregor AM. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N Engl J Med 1991;324:532–6.ArticlePubMed
- 48. Hume R, Simpson J, Delahunty C, van Toor H, Wu SY, Williams FL, et al. Human fetal and cord serum thyroid hormones: developmental trends and interrelationships. J Clin Endocrinol Metab 2004;89:4097–103.ArticlePubMed
- 49. Williams FL, Simpson J, Delahunty C, Ogston SA, Bongers-Schokking JJ, Murphy N, et al. Developmental trends in cord and postpartum serum thyroid hormones in preterm infants. J Clin Endocrinol Metab 2004;89:5314–20.ArticlePubMed
- 50. Persani L, Borgato S, Romoli R, Asteria C, Pizzocaro A, Beck-Peccoz P. Changes in the degree of sialylation of carbohydrate chains modify the biological properties of circulating thyrotropin isoforms in various physiological and pathological states. J Clin Endocrinol Metab 1998;83:2486–92.ArticlePubMed
- 51. Gasparoni P, Rubello D, Persani L, Beck-Peccoz P. Unusual association between a thyrotropin-secreting pituitary adenoma and a papillary thyroid carcinoma. Thyroid 1998;8:181–3.ArticlePubMed
- 52. Vulsma T, Gons MH, de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med 1989;321:13–6.ArticlePubMed
- 53. Fisher DA, Odell WD, Hobel CJ, Garza R. Thyroid function in the term fetus. Pediatrics 1969;44:526–35.PubMed
- 54. Legakis I, Adamopoulos D, Stamatiou I, Gryparis A, Chrousos GP. Divergent patterns of thyrotropin and other thyroidal parameters in relationship with the sex of healthy neonates and infants less than two years old: a longitudinal study. Thyroid 2019;29:920–7.ArticlePubMed
- 55. Chan MK, Seiden-Long I, Aytekin M, Quinn F, Ravalico T, Ambruster D, et al. Canadian Laboratory Initiative on Pediatric Reference Interval Database (CALIPER): pediatric reference intervals for an integrated clinical chemistry and immunoassay analyzer, Abbott ARCHITECT ci8200. Clin Biochem 2009;42:885–91.ArticlePubMed
- 56. Murphy N, Hume R, van Toor H, Matthews TG, Ogston SA, Wu SY, et al. The hypothalamic-pituitary-thyroid axis in preterm infants; changes in the first 24 hours of postnatal life. J Clin Endocrinol Metab 2004;89:2824–31.ArticlePubMed
- 57. Wide L, Eriksson K. Unique pattern of N-glycosylation, sialylation, and sulfonation on TSH molecules in serum of children up to 18 months. J Clin Endocrinol Metab 2019;104:4651–9.ArticlePubMed
- 58. Onsesveren I, Barjaktarovic M, Chaker L, de Rijke YB, Jaddoe VW, van Santen HM, et al. Childhood thyroid function reference ranges and determinants: a literature overview and a prospective cohort study. Thyroid 2017;27:1360–9.ArticlePubMed
- 59. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid 2014;24:1670–751.ArticlePubMedPMC
- 60. Campbell PJ, Brown SJ, Kendrew P, Lewer M, Lim EM, Joseph J, et al. Changes in thyroid function across adolescence: a longitudinal study. J Clin Endocrinol Metab 2020;105:dgz331.ArticlePubMed
- 61. Antalis CJ, Stevens LJ, Campbell M, Pazdro R, Ericson K, Burgess JR. Omega-3 fatty acid status in attention-deficit/ hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids 2006;75:299–308.ArticlePubMed
- 62. Wilken JA, Greenspan LC, Kushi LH, Voss RW, Windham GC. Thyroid hormones and timing of pubertal onset in a longitudinal cohort of females, Northern California, 2006-11. Paediatr Perinat Epidemiol 2016;30:285–93.ArticlePubMed
- 63. Gunapalasingham G, Frithioff-Bojsoe C, Lund MA, Hedley PL, Fonvig CE, Dahl M, et al. Reference values for fasting serum concentrations of thyroid-stimulating hormone and thyroid hormones in healthy Danish/North-European white children and adolescents. Scand J Clin Lab Invest 2019;79:129–35.ArticlePubMed
- 64. Mariotti S, Franceschi C, Cossarizza A, Pinchera A. The aging thyroid. Endocr Rev 1995;16:686–715.ArticlePubMed
- 65. Chahal HS, Drake WM. The endocrine system and ageing. J Pathol 2007;211:173–80.ArticlePubMed
- 66. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007;92:4575–82.ArticlePubMed
- 67. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489–99.ArticlePubMed
- 68. Bremner AP, Feddema P, Leedman PJ, Brown SJ, Beilby JP, Lim EM, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab 2012;97:1554–62.ArticlePubMed
- 69. Waring AC, Arnold AM, Newman AB, Buzkova P, Hirsch C, Cappola AR. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab 2012;97:3944–50.ArticlePubMedPMC
- 70. Chaker L, Korevaar TI, Medici M, Uitterlinden AG, Hofman A, Dehghan A, et al. Thyroid function characteristics and determinants: the Rotterdam Study. Thyroid 2016;26:1195–204.ArticlePubMed
- 71. Baloch Z, Carayon P, Conte-Devolx B, Demers LM, FeldtRasmussen U, Henry JF, et al. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003;13:3–126.ArticlePubMed
- 72. Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Lim EM, Walsh JP. The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 2013;98:2936–43.ArticlePubMed
- 73. Wehmann RE, Nisula BC. Radioimmunoassay of human thyrotropin: analytical and clinical developments. Crit Rev Clin Lab Sci 1984;20:243–83.ArticlePubMed
- 74. Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism: role of triiodothyronine in pituitary feedback in humans. N Engl J Med 1987;316:764–70.ArticlePubMed
- 75. Spencer CA, LoPresti JS, Patel A, Guttler RB, Eigen A, Shen D, et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab 1990;70:453–60.ArticlePubMed
- 76. Hoermann R, Eckl W, Hoermann C, Larisch R. Complex relationship between free thyroxine and TSH in the regulation of thyroid function. Eur J Endocrinol 2010;162:1123–9.ArticlePubMed
- 77. Clark PM, Holder RL, Haque SM, Hobbs FD, Roberts LM, Franklyn JA. The relationship between serum TSH and free T4 in older people. J Clin Pathol 2012;65:463–5.ArticlePubMed
- 78. Jansen SW, Akintola AA, Roelfsema F, van der Spoel E, Cobbaert CM, Ballieux BE, et al. Human longevity is characterised by high thyroid stimulating hormone secretion without altered energy metabolism. Sci Rep 2015;5:11525.ArticlePubMedPMC
- 79. Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab 2010;95:496–502.ArticlePubMed
- 80. Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005;90:5483–8.ArticlePubMed
- 81. Surks MI, Goswami G, Daniels GH. The thyrotropin reference range should remain unchanged. J Clin Endocrinol Metab 2005;90:5489–96.ArticlePubMed
- 82. Brabant G, Beck-Peccoz P, Jarzab B, Laurberg P, Orgiazzi J, Szabolcs I, et al. Is there a need to redefine the upper normal limit of TSH? Eur J Endocrinol 2006;154:633–7.ArticlePubMed
- 83. Boucai L, Hollowell JG, Surks MI. An approach for development of age-, gender-, and ethnicity-specific thyrotropin reference limits. Thyroid 2011;21:5–11.ArticlePubMedPMC
- 84. Kahapola-Arachchige KM, Hadlow N, Wardrop R, Lim EM, Walsh JP. Age-specific TSH reference ranges have minimal impact on the diagnosis of thyroid dysfunction. Clin Endocrinol (Oxf) 2012;77:773–9.ArticlePubMed
- 85. Vadiveloo T, Donnan PT, Murphy MJ, Leese GP. Age- and gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS). J Clin Endocrinol Metab 2013;98:1147–53.ArticlePubMed
- 86. Farrell CL, Nguyen L, Carter AC. Data mining for age-related TSH reference intervals in adulthood. Clin Chem Lab Med 2017;55:e213–5.ArticlePubMed
- 87. Mokhtar KM. TSH continuous reference intervals by indirect methods: a comparisons to partitioned reference intervals. Clin Biochem 2020;85:53–6.ArticlePubMed
- 88. Raverot V, Bonjour M, Abeillon du Payrat J, Perrin P, Roucher-Boulez F, Lasolle H, et al. Age- and sex-specific TSH upper-limit reference intervals in the general French population: there is a need to adjust our actual practices. J Clin Med 2020;9:792.ArticlePubMedPMC
- 89. Vesper HW, Van Uytfanghe K, Hishinuma A, Raverot V, Patru MM, Danilenko U, et al. Implementing reference systems for thyroid function tests: a collaborative effort. Clin Chim Acta 2021;519:183–6.ArticlePubMed
- 90. Meyerovitch J, Rotman-Pikielny P, Sherf M, Battat E, Levy Y, Surks MI. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med 2007;167:1533–8.ArticlePubMed
- 91. Taylor PN, Iqbal A, Minassian C, Sayers A, Draman MS, Greenwood R, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med 2014;174:32–9.ArticlePubMed
- 92. Livadas S, Bothou C, Androulakis I, Boniakos A, Angelopoulos N, Duntas L. Levothyroxine replacement therapy and overuse: a timely diagnostic approach. Thyroid 2018;28:1580–6.Article
- 93. Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab 2010;95:186–93.ArticlePubMed
- 94. Thayakaran R, Adderley NJ, Sainsbury C, Torlinska B, Boelaert K, Sumilo D, et al. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ 2019;366:l4892.ArticlePubMedPMC
- 95. Mooijaart SP, Du Puy RS, Stott DJ, Kearney PM, Rodondi N, Westendorp RG, et al. Association between levothyroxine treatment and thyroid-related symptoms among adults aged 80 years and older with subclinical hypothyroidism. JAMA 2019;322:1977–86.ArticlePubMedPMC
- 96. Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RG, Mooijaart SP, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017;376:2534–44.PubMed
- 97. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA 2004;292:2591–9.ArticlePubMed
- 98. Atzmon G, Barzilai N, Hollowell JG, Surks MI, Gabriely I. Extreme longevity is associated with increased serum thyrotropin. J Clin Endocrinol Metab 2009;94:1251–4.ArticlePubMedPMC
- 99. Atzmon G, Barzilai N, Surks MI, Gabriely I. Genetic predisposition to elevated serum thyrotropin is associated with exceptional longevity. J Clin Endocrinol Metab 2009;94:4768–75.ArticlePubMedPMC
- 100. Rozing MP, Houwing-Duistermaat JJ, Slagboom PE, Beekman M, Frolich M, de Craen AJ, et al. Familial longevity is associated with decreased thyroid function. J Clin Endocrinol Metab 2010;95:4979–84.ArticlePubMed
- 101. Pearce SH, Razvi S, Yadegarfar ME, Martin-Ruiz C, Kingston A, Collerton J, et al. Serum thyroid function, mortality and disability in advanced old age: the Newcastle 85+ Study. J Clin Endocrinol Metab 2016;101:4385–94.ArticlePubMedPMC
- 102. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010;304:1365–74.ArticlePubMedPMC
- 103. Moon S, Kim MJ, Yu JM, Yoo HJ, Park YJ. Subclinical hypothyroidism and the risk of cardiovascular disease and allcause mortality: a meta-analysis of prospective cohort studies. Thyroid 2018;28:1101–10.ArticlePubMed
- 104. Moon S, Kong SH, Choi HS, Hwangbo Y, Lee MK, Moon JH, et al. Relation of subclinical hypothyroidism is associated with cardiovascular events and all-cause mortality in adults with high cardiovascular risk. Am J Cardiol 2018;122:571–7.ArticlePubMed
- 105. Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012;172:811–7.ArticlePubMed
- 106. Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 2008;93:2998–3007.ArticlePubMed
- 107. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008;29:76–131.ArticlePubMed
- 108. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 2012;22:1200–35.ArticlePubMed
- 109. Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013;2:215–28.ArticlePubMedPMC
- 110. Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: a review. JAMA 2019;322:153–60.ArticlePubMed
- 111. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA 2019;322:1961–2.ArticlePubMed
References
Figure & Data
References
Citations

- The ageing thyroid: implications for longevity and patient care
Diana van Heemst
Nature Reviews Endocrinology.2024; 20(1): 5. CrossRef - Incidence and Determinants of Spontaneous Normalization of Subclinical Hypothyroidism in Older Adults
Evie van der Spoel, Nicolien A van Vliet, Rosalinde K E Poortvliet, Robert S Du Puy, Wendy P J den Elzen, Terence J Quinn, David J Stott, Naveed Sattar, Patricia M Kearney, Manuel R Blum, Heba Alwan, Nicolas Rodondi, Tinh-Hai Collet, Rudi G J Westendorp,
The Journal of Clinical Endocrinology & Metabolism.2024; 109(3): e1167. CrossRef - Multi-trait analysis characterizes the genetics of thyroid function and identifies causal associations with clinical implications
Rosalie B. T. M. Sterenborg, Inga Steinbrenner, Yong Li, Melissa N. Bujnis, Tatsuhiko Naito, Eirini Marouli, Tessel E. Galesloot, Oladapo Babajide, Laura Andreasen, Arne Astrup, Bjørn Olav Åsvold, Stefania Bandinelli, Marian Beekman, John P. Beilby, Jette
Nature Communications.2024;[Epub] CrossRef - Evaluation of multiple organophosphate insecticide exposure in relation to altered thyroid hormones in NHANES 2007‐2008 adult population
Massira Ousseni Diawara, Songtao Li, Mingzhi Zhang, Francis Manyori Bigambo, Xu Yang, Xu Wang, Tianyu Dong, Di Wu, Chenghao Yan, Yankai Xia
Ecotoxicology and Environmental Safety.2024; 273: 116139. CrossRef - Thyroid-function reference ranges in the diagnosis of thyroid dysfunction in adults
Salman Razvi
Nature Reviews Endocrinology.2024; 20(5): 253. CrossRef - Association between exposure to chemical mixtures and epigenetic ageing biomarkers: Modifying effects of thyroid hormones and physical activity
Wanying Shi, Jianlong Fang, Huimin Ren, Peijie Sun, Juan Liu, Fuchang Deng, Shuyi Zhang, Qiong Wang, Jiaonan Wang, Shilu Tong, Song Tang, Xiaoming Shi
Journal of Hazardous Materials.2024; 469: 134009. CrossRef - DNA Methylation in Autoimmune Thyroid Disease
Nicole Lafontaine, Scott G Wilson, John P Walsh
The Journal of Clinical Endocrinology & Metabolism.2023; 108(3): 604. CrossRef - A Causality between Thyroid Function and Bone Mineral Density in Childhood: Abnormal Thyrotropin May Be Another Pediatric Predictor of Bone Fragility
Dongjin Lee, Moon Ahn
Metabolites.2023; 13(3): 372. CrossRef - Serum Lipidomic Analysis Reveals Biomarkers and Metabolic Pathways of Thyroid Dysfunction
Hua Dong, Wenjie Zhou, Xingxu Yan, Huan Zhao, Honggang Zhao, Yan Jiao, Guijiang Sun, Yubo Li, Zuncheng Zhang
ACS Omega.2023; 8(11): 10355. CrossRef - Developmental and environmental modulation of fecal thyroid hormone levels in wild Assamese macaques (Macaca assamensis)
Verena Behringer, Michael Heistermann, Suchinda Malaivijitnond, Oliver Schülke, Julia Ostner
American Journal of Primatology.2023;[Epub] CrossRef - Prevalence of Functional Alterations and the Effects of Thyroid
Autoimmunity on the Levels of TSH in an Urban Population of Colombia:
A Population-Based Study
Hernando Vargas-Uricoechea, Valentina Agredo-Delgado, Hernando David Vargas-Sierra, María V. Pinzón-Fernández
Endocrine, Metabolic & Immune Disorders - Drug Targets.2023; 23(6): 857. CrossRef - Genetic determinants of thyroid function in children
Tessa A Mulder, Purdey J Campbell, Peter N Taylor, Robin P Peeters, Scott G Wilson, Marco Medici, Colin Dayan, Vincent V W Jaddoe, John P Walsh, Nicholas G Martin, Henning Tiemeier, Tim I M Korevaar
European Journal of Endocrinology.2023; 189(2): 164. CrossRef - Relationship between Thyroid CT Density, Volume, and Future TSH Elevation: A 5-Year Follow-Up Study
Tomohiro Kikuchi, Shouhei Hanaoka, Takahiro Nakao, Yukihiro Nomura, Takeharu Yoshikawa, Md Ashraful Alam, Harushi Mori, Naoto Hayashi
Life.2023; 13(12): 2303. CrossRef - Thyroid Stimulating Hormone and Thyroid Hormones (Triiodothyronine and Thyroxine): An American Thyroid Association-Commissioned Review of Current Clinical and Laboratory Status
Katleen Van Uytfanghe, Joel Ehrenkranz, David Halsall, Kelly Hoff, Tze Ping Loh, Carole A. Spencer, Josef Köhrle
Thyroid®.2023; 33(9): 1013. CrossRef - Blood hormones and suicidal behaviour: A systematic review and meta-analysis
Xue-Lei Fu, Xia Li, Jia-Mei Ji, Hua Wu, Hong-Lin Chen
Neuroscience & Biobehavioral Reviews.2022; 139: 104725. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite