Articles
- Page Path
- HOME > Endocrinol Metab > Volume 29(4); 2014 > Article
-
Original ArticleClinical Study Increased Risk of Diabetes Development in Subjects with the Hypertriglyceridemic Waist Phenotype: A 4-Year Longitudinal Study
- Ki Joong Han, Shin Yeoung Lee, Nam Hee Kim, Hyun Beom Chae, Tae Hoon Lee, Choel Min Jang, Kyung Mo Yoo, Hae Jung Park, Min Kyung Lee, Won Seon Jeon, Se Eun Park, Cheol-Young Park, Won-Young Lee, Ki-Won Oh, Sung-Woo Park, Eun-Jung Rhee
-
Endocrinology and Metabolism 2014;29(4):514-521.
DOI: https://doi.org/10.3803/EnM.2014.29.4.514
Published online: December 29, 2014
Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding author: Eun-Jung Rhee. Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 110-746, Korea. Tel: +82-2-2001-2485, Fax: +82-2-2001-1588, hongsiri@hanmail.net
Copyright © 2014 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- The hypertriglyceridemic waist (HTGW) phenotype is a simple and inexpensive screening parameter to identify people at increased risk of cardiovascular disease. We evaluated whether the HTGW phenotype predicts diabetes in urban Korean adults.
-
Methods
- A total of 2,900 nondiabetic subjects (mean age 44.3 years), comprising 2,078 males (71.7%) and 822 females (28.3%) who underwent annual medical check-ups at our center between January 2005 and December 2009, were recruited. The subjects were divided into four groups according to baseline serum triglyceride (TG) level and waist circumference (WC): normal WC-normal TG (NWNT) level, normal WC-high TG level, enlarged WC-normal TG level, and enlarged WC-high TG (EWHT) level. High serum TG level was defined as ≥150 mg/dL and enlarged WC was defined as ≥90 cm for men and ≥85 cm for women. New cases of diabetes were determined according to questionnaires filled in by participants and the diagnostic criteria of the American Diabetes Association. Cox proportional hazards model analysis was used to assess the association of HTGW phenotype with the incidence of diabetes.
-
Results
- A total of 101 (3.5%) new diabetes cases were diagnosed during the study period. The EWHT group had a higher incidence of diabetes (8.3%) compared with the NWNT group (2.2%). The adjusted hazard ratio for diabetes for subjects with the EWHT phenotype at baseline was 4.113 (95% confidence interval [CI], 2.397 to 7.059) after adjustment for age, and 2.429 (95% CI, 1.370 to 4.307) after adjustment for age, sex, total cholesterol, systolic blood pressure, and alcohol drinking history. It was attenuated by inclusion of baseline fasting glucose level in the model.
-
Conclusion
- Subjects with the HTGW phenotype showed the highest risk of incident diabetes. This tool could be useful for identifying individuals at high risk of diabetes.
- Diabetes has become one of the major public-health challenges worldwide, owing to a westernized lifestyle [1]. Diabetes and its complications have become a major cause of morbidity and mortality in most developing and developed countries. Recently, the International Diabetes Federation (IDF) estimated that in 2011 there were 366 million people worldwide with diabetes, and predicted that this figure will rise to 552 million by 2030 [1]. As diabetes becomes more prevalent, its social and economic costs will become one of the major problems for patients and society [2,3]. To slow the markedly increasing rate of diabetes, it is important to identify and treat risk factors for diabetes.
- The pathogenesis of type 2 diabetes mellitus (T2DM) is heterogeneous, and both genetic factors affecting insulin release and responsiveness and environmental factors, such as obesity, are important for its development. In 1988, Reaven [4] proposed that insulin resistance is a fundamental disorder associated with a cluster of diabetogenic, atherogenic, prothrombotic, and inflammatory metabolic abnormalities now called metabolic syndrome (MetS). Recently, the criteria for MetS from the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) [5] and IDF [6] are most widely used as screening tools to identify subjects with features of MetS. The MetS definition proposed by the IDF acknowledges the importance of central obesity in MetS by making it a required component for clinical diagnosis.
- As waist circumference (WC) cannot fully discriminate visceral adiposity from subcutaneous abdominal adiposity, elevated triglyceride (TG) levels have been adopted as a marker of dysfunctional visceral adipose tissue [7,8,9]. Lemieux et al. [9] were the first group to recognize that the hypertriglyceridemia plus large WC (hypertriglyceridemic waist, HTGW) phenotype is associated with increased cardiovascular disease (CVD) risk. In particular, the HTGW is associated with the atherogenic triad of hyperinsulinemia, elevated concentrations of apolipoprotein B and small, dense low density lipoprotein cholesterol (sdLDL-C) particles. Lemieux et al. [9] also suggested that visceral obesity and the HTGW phenotype are the central components of MetS. Recent evidence now indicates that MetS begins with excess central adiposity [10]. Therefore, the HTGW phenotype could be used as a simple and inexpensive screening tool to identify people at increased risk of MetS, T2DM, and CVD [9,11,12,13,14].
- As most of previous studies were performed in only Caucasians, we designed this study to compare the risk for diabetes development among the groups divided by HTGW phenotypes with a median follow-up of 48.7 months in a large cohort of nondiabetic Korean subjects who participated in a medical check-up program.
INTRODUCTION
- Subjects
- This was a retrospective study, and the subjects were participants in the Kangbuk Samsung Health Study, a large database of participants in a medical health check-up program at the Health Promotion Center of Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. The purpose of the medical health check-up program is to promote the health of employees through regular health check-ups and to enhance early detection of existing diseases. Most of the examinees are employees of various industrial companies from all around the country and their family members. The costs of the medical examinations are largely paid for by their employers, and a considerable proportion of the examinees undergo examinations annually or biannually.
- Initial data were obtained from 10,868 subjects in whom annual health check-ups were performed for 5 consecutive years between January 2005 and December 2009. Among these subjects, 7,968 were excluded due to the presence of diabetes or missing data, especially for WC and lipid profiles. The final analyses were performed in 2,900 subjects (2,078 men [71.7%] and 822 women [28.3%]) with a mean age of 44.3 years.
- The participants provided their written informed consent for the usage of the medical check-up data for the research. The design, protocol and the consent procedure of this study were reviewed and approved by Institutional Review Board of Kangbuk Samsung Hospital (KBS12089) and is in accordance with the Helsinki Declaration of 1975.
- Anthropometric and laboratory measurements
- Height and weight were measured twice and then averaged. Body mass index (BMI) was calculated by dividing the weight (kg) by the square of the height (m). Blood pressure (BP) was measured using a standardized sphygmomanometer after 5 minutes of rest. The WC was measured in the standing position, at the middle point between anterior iliac crest and the lower border of the ribs by a single examiner. Values for WC were only available for 2,900 subjects due to inconsistency of the measurement method.
- All of the subjects were examined after an overnight fast. The hexokinase method was used to measure fasting glucose concentrations (Hitachi Modular D2400, Roche, Tokyo, Japan). Fasting serum insulin concentrations were determined by electrochemiluminescence immunoassay (Hitachi Modular E170, Roche). An enzymatic calorimetric test was used to measure serum total cholesterol (TC) and TG concentrations. The selective inhibition method was used to measure the level of high density lipoprotein cholesterol (HDL-C), and a homogeneous enzymatic calorimetric test was used to measure the level of LDL-C. HbA1c was measured by immunoturbidimetric assay with a Cobra Integra 800 automatic analyzer (Roche Diagnostics, Basel, Switzerland) with a reference value of 4.4% to 6.4%. The methodology was aligned with the Diabetes Control and Complications Trial and National Glycohemoglobin Standardization Program (NGSP) standards [15]. The intra-assay coefficient of variation (CV) was 2.3% and the interassay CV was 2.4%; both were within the NGSP acceptable limits [16].
- Subjects with underlying diabetes at baseline were excluded from the study. The presence of diabetes mellitus was determined according to questionnaires filled in by participants and the diagnostic criteria of the American Diabetes Association [17]. Development of diabetes was assessed in each year's examination with the same diagnostic criteria for diabetes mellitus. HOMA-IR was calculated as follows: HOMA-IR=[fasting insulin (IU/mL)×fasting glucose (mmol/L)]/22.5 [18].
- The presence of hypertension was defined by criteria recommended by the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) [19]: BP ≥140/90 mm Hg or current antihypertensive medication use. Alcohol drinking was defined by drinking more than three times a week in the self-administered questionnaire.
- Diagnosis of MetS was based on two criteria:
- 1) Modified NCEP-ATP III [5]: three or more of the following components: (1) abdominal obesity: WC ≥90 cm in men, ≥85 cm in women [20]; (2) elevated blood pressure: systolic blood pressure (SBP) ≥130 mm Hg or diastolic blood pressure (DBP) ≥85 mm Hg, or treatment for previously diagnosed hypertension; (3) elevated fasting plasma glucose: ≥100 mg/dL (≥5.6 mmol/L) or previously diagnosed type 2 diabetes; (4) hypertriglyceridemia: TG ≥150 mg/dL (≥1.7 mmol/L); (5) reduced HDL-C: <40 mg/dL (<1.03 mmol/L) in men and <50 mg/dL (<1.29 mmol/L) in women.
- 2) IDF [6]: the presence of abdominal obesity (WC ≥90 cm in men, ≥85 cm in women [20]) plus two of following components: (1) elevated blood pressure: SBP ≥130 mm Hg or DBP ≥85 mm Hg, or treatment for previously diagnosed hypertension; (2) elevated fasting plasma glucose: ≥100 mg/dL (≥5.6 mmol/L) or previously diagnosed type 2 diabetes; (3) hypertriglyceridemia: TG ≥150 mg/dL (≥1.7 mmol/L); (4) reduced HDL-C: <40 mg/dL (<1.03 mmol/L) in men and <50 mg/dL (<1.29 mmol/L) in women.
- HTGW was diagnosed using anthropometric criteria for the Korean population [19] (WC: ≥90 cm for men and ≥85 cm for women) and fasting plasma TG ≥150 mg/dL.
- According to the above criteria, participants were divided into four groups: 1) normal WC, normal TG (NWNT): WC <90 cm in men, WC <85 cm in women, and TG <150 mg/dL; 2) normal WC, high TG (NWHT): WC <90 cm in men, WC <85 cm in women, and TG ≥150 mg/dL; 3) enlarged WC, normal TG (EWNT): WC ≥90 cm in men, WC ≥85 cm in women, and TG <150 mg/dL; 4) enlarged WC, high TG (EWHT): WC ≥90 cm in men, WC ≥85 cm in women, and TG ≥150 mg/dL.
- Statistical analysis
- All data were analyzed using SPSS version 18.0 (IBM Co., Armonk, NY, USA). Comparisons of the variables among the four groups divided by baseline TG level and WC were performed by one-way analysis of variance. Data that did not follow a normal distribution were analyzed after logarithmic transformation. Comparisons of adjusted hazard ratios (aHRs) for incident diabetes in the four groups divided by baseline TG level and WC were analyzed by Cox proportional hazards model analysis after adjustment for confounding baseline variables. Kaplan-Meier survival analyses were performed for incident diabetes for 4 years according to baseline TG level and WC. Statistical significance was defined as P<0.05.
METHODS
- Study population and baseline characteristics
- The mean age of the participants was 44.3 years (Table 1). At baseline, 1,790 subjects (61.7%) were in the NWNT group, 615 (21.2%) in the NWHT group, 255 (8.8%) in the EWNT group, and 240 (8.3%) in the EWHT group.
- Anthropometric characteristics and metabolic risk profiles of subjects with or without MetS defined by NCEP-ATP III criteria, IDF criteria, and HTGW phenotype are presented in Table 2. The prevalence of MetS defined by NCEP-ATP III criteria, IDF criteria, and HTGW phenotype was 21.0%, 10.9%, and 8.3%, respectively. Values for adiposity indices (BMI and WC) were higher in subjects satisfying the MetS criteria compared with subjects with the HTGW phenotype, irrespective of the definition used (P<0.01). Subjects with the HTGW phenotype displayed worse lipid profiles, including lower HDL-C levels, higher TG levels, and higher fasting glucose concentrations, compared with subjects without this phenotype (P<0.05). Similar results were shown in subjects with MetS defined by NCEP-ATP III and IDF criteria (P<0.01). The lipid profiles showed similar findings among subjects who met the NCEP-ATP III and IDF criteria and who had the HTGW phenotype.
- Hazard ratios for diabetes according to the groups divided by baseline WC and TG level
- During a median follow-up of 48.7 months, 101 subjects (3.5%) developed diabetes. Incidence rates for diabetes were 2.2%, 4.1%, 6.7%, and 8.3% in the NWNT, NWHT, EWNT, and EWHT groups, respectively (Table 1).
- In a Cox proportional hazards model with diabetes development as the dependent variable, EWHT subjects had an aHR of 4.113 (95% confidence interval [CI], 2.397 to 7.059), EWNT subjects an aHR of 2.866 (95% CI, 1.616 to 5.083), and NWHT subjects an aHR of 1.942 (95% CI, 1.175 to 3.211) for diabetes after adjustment for age, with the NWNT group as the reference group (Table 3). Similar results were observed in model 2 (adjusted for age, sex, TC, SBP, and alcohol drinking history). In model 2, the aHR for diabetes was 2.429 (95% CI, 1.370 to 4.307) in the EWHT group, 2.390 (95% CI, 1.346 to 4.245) in the EWNT group and 1.328 (95% CI, 0.783 to 2.251) in the NWHT group, with the NWNT group as the reference group. When baseline fasting glucose level was also included in the model (model 3), the risks were attenuated, with an aHR of 2.251 in the EWNT group and 1.569 in the EWHT group (Table 3). There was no interaction between serum TG level and WC for the development of diabetes (P=0.238).
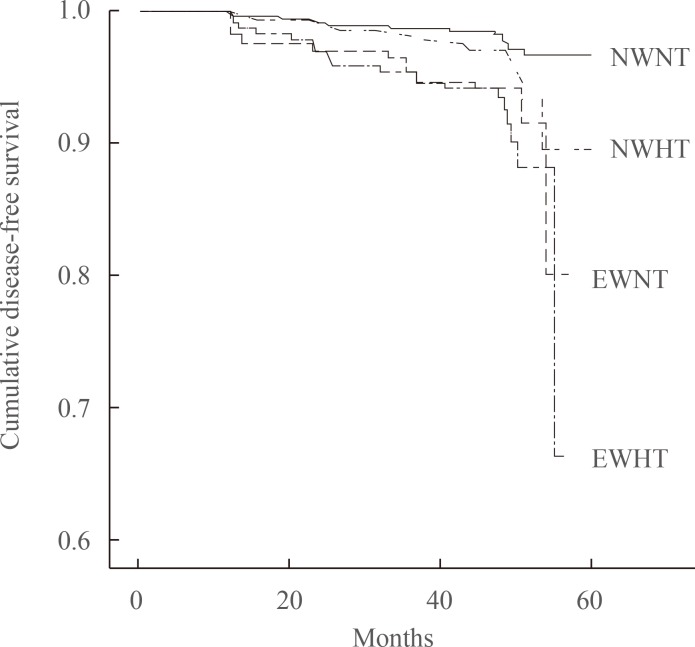
- In a Kaplan-Meier disease-free survival analysis, the EWHT group showed the lowest disease-free survival for diabetes among the four groups. The EWNT group showed the second lowest disease-free survival (Fig. 1). The NWNT group showed the highest disease-free survival among the groups.
RESULTS
- In this study, the EWHT group showed a significantly increased risk of diabetes development compared with the NWNT group during a median of 48.7 months of follow-up. In a Cox proportional hazards model, the EWHT group showed a significantly higher aHR for diabetes compared with the NWNT group, with a 4.1-fold increased risk in model 1 and a 2.4-fold increased risk in model 2. However, when baseline fasting glucose was included in the model, these effects were attenuated, suggesting the importance of fasting glucose in the development of diabetes. High TG levels and enlarged WC could have affected diabetes development through development of insulin resistance.
- It is known that adipose tissue is not only an accumulation of fat cells but is also an active endocrine organ that secretes various adipocytokines that influence energy expenditure and metabolism in our body [21]. Furthermore, where adipose tissue is deposited affects metabolic function more than the actual amount. For example, visceral fat is the starting point for insulin resistance as it is more prone to lipolysis and releases free fatty acids into the circulation, whereas subcutaneous fat is known to protect against insulin resistance and obesity [22].
- MetS is a cluster of various risk factors for CVDs [23]. Although multiple risk factors are included in the definition of MetS, the concept of MetS stems from insulin resistance and abdominal obesity. However, obesity is remarkably heterogeneous. Some very obese patients have a fairly normal metabolic risk profile, despite their obesity. On the other hand, some moderately overweight individuals have a whole cluster of atherogenic and diabetogenic metabolic abnormalities. The recent concept of 'metabolically healthy obesity' refers to the subgroup of obese subjects who are metabolically healthy and protected from CVDs [24,25,26].
- In 2000, Lemieux et al. [8] suggested the concept of the 'HTGW' phenotype as the simultaneous presence of an elevated fasting TG level and an enlarged WC. They found that a moderately or substantially enlarged WC (WC ≥90 cm but <100 cm and WC >100 cm) in the absence of an elevated TG level was not enough to adequately discriminate men with the metabolic triad (i.e., elevated fasting insulin and apo B concentrations and sdLDL-C particles). Therefore, they concluded that an enlarged WC alone is not enough to identify men with the atherogenic metabolic triad and that considering the presence of a hypertriglyceridemia could further improve the screening procedure. They suggested that the identification of subjects with the HTGW phenotype improves the selection of subjects at high risk of metabolic disturbances, insulin resistance and subsequent CVD [9]. However, we found that high TG level and enlarged WC independently increased the risk of diabetes, since there was no interaction between those two factors for the development of diabetes.
- Few studies have investigated the relationship between diabetes development and the HTGW phenotype in Asian populations. In a very recent retrospective study performed in 687 Chinese who were followed up for 15 years, the HWHT phenotype was associated with a 4.1-fold increased risk of diabetes, and presence of MetS was associated with a 3.7-fold increased risk of diabetes. In addition, for the population without elevated fasting plasma glucose levels, a multivariate analysis showed that the HTGW phenotype was associated with a 3.9-fold increased risk of diabetes and that presence of MetS was associated with a 3.7-fold increased risk of diabetes [27]. This is in line with our study results in that subjects with high WCs and hypertriglyceridemia showed a higher risk of diabetes compared with subjects with normal WCs and normal serum TG levels, suggesting the importance of visceral obesity and insulin resistance assessed by this simple and inexpensive method. However, when fasting glucose was included in the model, the risks were attenuated. As the previous study performed in Chinese did not analyze the results with adjustment for fasting blood glucose, our result is more reliable regarding the association between the HTGW phenotype and diabetes development.
- Our study has limitations. As it is observational, the precise mechanism for the results could not be fully explained. The lack of post-challenge glucose levels in the diagnosis of diabetes could have biased the true proportion of diabetes patients. However, as we included HbA1c ≥6.5% and history of diabetes medication use in the definition of diabetes, we had sufficient power to exclude subjects with diabetes [28]. Despite these limitations, the study is meaningful in that it is the first retrospective study regarding this issue performed in a large Korean urban population.
- In conclusion, the risk of diabetes development was higher in EWHT subjects compared with NWNT subjects. We should make efforts to improve the metabolic health of these high-risk subjects, and to initiate early intensive lifestyle modification in these subjects, as simple weight loss might not be the optimal solution for them.
DISCUSSION
- 1. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–321. ArticlePubMed
- 2. Lee KW. Costs of diabetes mellitus in Korea. Diabetes Metab J 2011;35:567–570. ArticlePubMedPMC
- 3. Centers for Disease Control and Prevention. 2011 National diabetes fact sheet [Internet]; Atlanta: Centers for Disease Control and Prevention; 2011. updated 2014 Jul 7. cited 2014 Aug 26. Available from: http://www.cdc.gov/diabetes/pubs/factsheet11.htm.
- 4. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607. ArticlePubMed
- 5. Assmann G, Guerra R, Fox G, Cullen P, Schulte H, Willett D, Grundy SM. Harmonizing the definition of the metabolic syndrome: comparison of the criteria of the Adult Treatment Panel III and the International Diabetes Federation in United States American and European populations. Am J Cardiol 2007;99:541–548. ArticlePubMed
- 6. Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome: a new worldwide definition. Lancet 2005;366:1059–1062. ArticlePubMed
- 7. Sam S, Haffner S, Davidson MH, D'Agostino RB Sr, Feinstein S, Kondos G, Perez A, Mazzone T. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diabetes Care 2009;32:1916–1920. ArticlePubMedPMC
- 8. Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Almeras N, Bergeron J, Gaudet D, Tremblay G, Prud'homme D, Nadeau A, Despres JP. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation 2000;102:179–184. ArticlePubMed
- 9. Lemieux I, Poirier P, Bergeron J, Almeras N, Lamarche B, Cantin B, Dagenais GR, Despres JP. Hypertriglyceridemic waist: a useful screening phenotype in preventive cardiology? Can J Cardiol 2007;23(Suppl B):23B–31B.ArticlePubMedPMC
- 10. Cameron AJ, Boyko EJ, Sicree RA, Zimmet PZ, Söderberg S, Alberti KG, Tuomilehto J, Chitson P, Shaw JE. Central obesity as a precursor to the metabolic syndrome in the AusDiab study and Mauritius. Obesity (Silver Spring) 2008;16:2707–2716. ArticlePubMed
- 11. Bos G, Dekker JM, Heine RJ. Hoom study. Non-HDL cholesterol contributes to the "hypertriglyceridemic waist" as a cardiovascular risk factor: the Hoorn study. Diabetes Care 2004;27:283–284. ArticlePubMed
- 12. LaMonte MJ, Ainsworth BE, DuBose KD, Grandjean PW, Davis PG, Yanowitz FG, Durstine JL. The hypertriglyceridemic waist phenotype among women. Atherosclerosis 2003;171:123–130. ArticlePubMed
- 13. Solati M, Ghanbarian A, Rahmani M, Sarbazi N, Allahverdian S, Azizi F. Cardiovascular risk factors in males with hypertriglycemic waist (Tehran Lipid and Glucose Study). Int J Obes Relat Metab Disord 2004;28:706–709. ArticlePubMedPDF
- 14. Lemieux I, Almeras N, Mauriege P, Blanchet C, Dewailly E, Bergeron J, Despres JP. Prevalence of 'hypertriglyceridemic waist' in men who participated in the Quebec Health Survey: association with atherogenic and diabetogenic metabolic risk factors. Can J Cardiol 2002;18:725–732. PubMed
- 15. NGSP. List of NGSP certified methods [Internet]; [place unknown]: NGSP; c2010. cited 2012 Nov 26. Available from: http://www.ngsp.org/docs/methods.pdf.
- 16. Schwartz KL, Monsur JC, Bartoces MG, West PA, Neale AV. Correlation of same-visit HbA1c test with laboratory-based measurements: a MetroNet study. BMC Fam Pract 2005;6:28ArticlePubMedPMCPDF
- 17. American Diabetes Association. Standards of medical care in diabetes: 2013. Diabetes Care 2013;36(Suppl 1):S11–S66. ArticlePubMed
- 18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. ArticlePubMedPDF
- 19. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–2572. ArticlePubMed
- 20. Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 2007;75:72–80. ArticlePubMed
- 21. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010;316:129–139. ArticlePubMed
- 22. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–887. ArticlePubMed
- 23. Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am 2014;43:1–23. ArticlePubMed
- 24. Bluher M. Are there still healthy obese patients? Curr Opin Endocrinol Diabetes Obes 2012;19:341–346. ArticlePubMed
- 25. Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab 2012;97:2482–2488. ArticlePubMedPMC
- 26. Rhee EJ, Seo MH, Kim JD, Jeon WS, Park SE, Park CY, Oh KW, Park SW, Lee WY. Metabolic health is more closely associated with coronary artery calcification than obesity. PLoS One 2013;8:e74564ArticlePubMedPMC
- 27. He S, Zheng Y, Shu Y, He J, Wang Y, Chen X. Hypertriglyceridemic waist might be an alternative to metabolic syndrome for predicting future diabetes mellitus. PLoS One 2013;8:e73292ArticlePubMedPMC
- 28. Alqahtani N, Khan WA, Alhumaidi MH, Ahmed YA. Use of glycated hemoglobin in the diagnosis of diabetes mellitus and pre-diabetes and role of fasting plasma glucose, oral glucose tolerance test. Int J Prev Med 2013;4:1025–1029. PubMedPMC
References

Values are expressed as mean±SD or number (%).
NWNT, normal waist with normal plasma triglyceride level; NWHT, normal waist with hypertriglyceridemia; EWNT, enlarged waist with normal plasma triglyceride level; EWHT, enlarged waist with hypertriglyceridemia; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood glucose; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; HbA1c, glycated hemoglobin; HOMA-IR, homeostasis model assessment-insulin resistance.

Values are expressed as mean±SD or number (%).
NCEP-ATP III, National Cholesterol Education Program-Adult Treatment Panel III; IDF, International Diabetes Federation; HTGW, hypertriglyceridemic waist; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood glucose; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; HbA1c, glycated hemoglobin; HOMA-IR, homeostasis model assessment-insulin resistance.
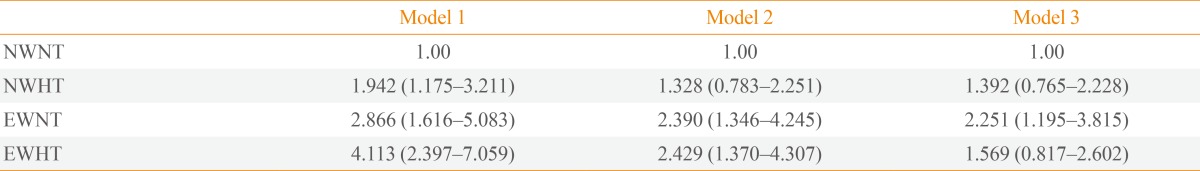
Model 1 was adjusted for age. Model 2 was adjusted for age, sex, total cholesterol, systolic blood pressure, and alcohol drinking history. Model 3 was adjusted for the factors adjusted for in model 2 plus fasting glucose level at baseline.
NWNT, normal waist, normal triglyceride level; NWHT, normal waist, hypertriglyceridemia; EWNT, enlarged waist, normal triglyceride level; EWHT, enlarged waist, hypertriglyceridemia.
Figure & Data
References
Citations

- Triglyceridemic Waist Phenotypes as Risk Factors for Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
Fiorella E. Zuzunaga-Montoya, Víctor Juan Vera-Ponce
International Journal of Statistics in Medical Research.2024; 13: 19. CrossRef - Association between hypertriglyceridemic-waist phenotype and circadian syndrome risk: a longitudinal cohort study
Li-Kun Hu, Yu-Hong Liu, Kun Yang, Ning Chen, Lin-Lin Ma, Yu-Xiang Yan
Hormones.2023; 22(3): 457. CrossRef - Caracterización del fenotipo de cintura hipertrigliceridémica en pacientes con diabetes mellitus tipo 2 en España: un estudio epidemiológico
I. Miñambres, J. Sánchez-Hernández, G. Cuixart, A. Sánchez-Pinto, J. Sarroca, A. Pérez
Revista Clínica Española.2021; 221(10): 576. CrossRef - Association of “hypertriglyceridemic waist” with increased 5-year risk of subclinical atherosclerosis in a multi-ethnic population: a prospective cohort study
Peyman Namdarimoghaddam, Adeleke Fowokan, Karin H. Humphries, G. B. John Mancini, Scott Lear
BMC Cardiovascular Disorders.2021;[Epub] CrossRef - Characterization of the hypertriglyceridemic waist phenotype in patients with type 2 diabetes mellitus in Spain: an epidemiological study
I. Miñambres, J. Sánchez-Hernandez, G. Cuixart, A. Sánchez-Pinto, J. Sarroca, A. Pérez
Revista Clínica Española (English Edition).2021; 221(10): 576. CrossRef - Association between Hypertriglyceridemic-Waist Phenotype and Risk of Type 2 Diabetes Mellitus in Middle-Aged and Older Chinese Population: A Longitudinal Cohort Study
Dezhong Chen, Ziyun Liang, Huimin Sun, Ciyong Lu, Weiqing Chen, Harry H. X. Wang, Vivian Yawei Guo
International Journal of Environmental Research and Public Health.2021; 18(18): 9618. CrossRef - Metabolic Syndrome, and Particularly the Hypertriglyceridemic-Waist Phenotype, Increases Breast Cancer Risk, and Adiponectin Is a Potential Mechanism: A Case–Control Study in Chinese Women
Yujuan Xiang, Wenzhong Zhou, Xuening Duan, Zhimin Fan, Shu Wang, Shuchen Liu, Liyuan Liu, Fei Wang, Lixiang Yu, Fei Zhou, Shuya Huang, Liang Li, Qiang Zhang, Qinye Fu, Zhongbing Ma, Dezong Gao, Shude Cui, Cuizhi Geng, Xuchen Cao, Zhenlin Yang, Xiang Wang,
Frontiers in Endocrinology.2020;[Epub] CrossRef - Hypertriglyceridemic Waist Phenotype and Lipid Accumulation Product: Two Comprehensive Obese Indicators of Waist Circumference and Triglyceride to Predict Type 2 Diabetes Mellitus in Chinese Population
Minrui Xu, Mingtao Huang, Deren Qiang, Jianxin Gu, Yong Li, Yingzi Pan, Xingjuan Yao, Wenchao Xu, Yuan Tao, Yihong Zhou, Hongxia Ma, Ulrike Rothe
Journal of Diabetes Research.2020; 2020: 1. CrossRef - Prevalence and relationship of hypertriglyceridaemic–waist phenotype and type 2 diabetes mellitus among a rural adult Chinese population
Yong-Cheng Ren, Yu Liu, Xi-Zhuo Sun, Bing-Yuan Wang, Yi Liu, Hu Ni, Yang Zhao, Dechen Liu, Xuejiao Liu, Dongdong Zhang, Feiyan Liu, Cheng Cheng, Leilei Liu, Xu Chen, Qionggui Zhou, Ming Zhang, Dongsheng Hu
Public Health Nutrition.2019; 22(8): 1361. CrossRef - Hypertriglyceridemic waist phenotype and abnormal glucose metabolism: a system review and meta-analysis
Chun-Ming Ma, Xiao-Li Liu, Na Lu, Rui Wang, Qiang Lu, Fu-Zai Yin
Endocrine.2019; 64(3): 469. CrossRef - Superior Role of Waist Circumference to Body-Mass Index in the Prediction of Cardiometabolic Risk in Dyslipidemic Patients
Ľ. Cibičková, K. Langová, H. Vaverková, J. Lukeš, N. Cibiček
Physiological Research.2019; : 931. CrossRef - Being Metabolically Healthy, the Most Responsible Factor for Vascular Health
Eun-Jung Rhee
Diabetes & Metabolism Journal.2018; 42(1): 19. CrossRef - Letter: Utility of the Visceral Adiposity Index and Hypertriglyceridemic Waist Phenotype for Predicting Incident Hypertension (Endocrinol Metab 2017;32:221-9, Mohsen Janghorbani et al.)
Eun-Jung Rhee
Endocrinology and Metabolism.2017; 32(3): 396. CrossRef - The Relationship between Hypertriglyceridemic Waist Phenotype and Early Diabetic Nephropathy in Type 2 Diabetes
Chun-Ming Ma, Rui Wang, Xiao-Li Liu, Na Lu, Qiang Lu, Fu-Zai Yin
Cardiorenal Medicine.2017; 7(4): 295. CrossRef - The Association of Hypertriglyceridemic Waist Phenotype with Chronic Kidney Disease and Its Sex Difference: A Cross-Sectional Study in an Urban Chinese Elderly Population
Jing Zeng, Miao Liu, Lei Wu, Jianhua Wang, Shanshan Yang, Yiyan Wang, Yao Yao, Bin Jiang, Yao He
International Journal of Environmental Research and Public Health.2016; 13(12): 1233. CrossRef - Prevalence of hypertriglyceridemic waist and association with risk of type 2 diabetes mellitus: a meta‐analysis
Yongcheng Ren, Xinping Luo, Chongjian Wang, Lei Yin, Chao Pang, Tianping Feng, Bingyuan Wang, Lu Zhang, Linlin Li, Xiangyu Yang, Hongyan Zhang, Jingzhi Zhao, Dongsheng Hu
Diabetes/Metabolism Research and Reviews.2016; 32(4): 405. CrossRef - Utility of hypertriglyceridemic waist phenotype for predicting incident type 2 diabetes: The Isfahan Diabetes Prevention Study
Mohsen Janghorbani, Masoud Amini
Journal of Diabetes Investigation.2016; 7(6): 860. CrossRef - βig-h3 Represses T-Cell Activation in Type 1 Diabetes
Maeva Patry, Romain Teinturier, Delphine Goehrig, Cornelia Zetu, Doriane Ripoche, In-San Kim, Philippe Bertolino, Ana Hennino
Diabetes.2015; 64(12): 4212. CrossRef - Hypertriglyceridemic Waist – a Simple Clinical Tool to Detect Cardiometabolic Risk: Comparison With Harmonized Definition of Metabolic Syndrome
H. VAVERKOVÁ, D. KARÁSEK, D. NOVOTNÝ, M. HALENKA, J. ORSÁG, L. SLAVÍK
Physiological Research.2015; : S385. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef - Changes in Metabolic Health Status Over Time and Risk of Developing Type 2 Diabetes
Seung-Hwan Lee, Hae Kyung Yang, Hee-Sung Ha, Jin-Hee Lee, Hyuk-Sang Kwon, Yong-Moon Park, Hyeon-Woo Yim, Moo-Il Kang, Won-Chul Lee, Ho-Young Son, Kun-Ho Yoon
Medicine.2015; 94(40): e1705. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite


