Articles
- Page Path
- HOME > Endocrinol Metab > Volume 29(2); 2014 > Article
-
Review ArticleTranscriptional Regulation of Fibroblast Growth Factor 21 Expression
- Kwi-Hyun Bae, Jung-Guk Kim, Keun-Gyu Park
-
Endocrinology and Metabolism 2014;29(2):105-111.
DOI: https://doi.org/10.3803/EnM.2014.29.2.105
Published online: June 26, 2014
Division of Endocrinology and Metabolism, Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- Corresponding author: Keun-Gyu Park. Department of Internal Medicine, Kyungpook National University Hospital, Kyungpook National University School of Medicine, 130 Dongdeok-ro, Jung-gu, Daegu 700-721, Korea. Tel: +82-53-200-3159, Fax: +82-53-426-6722, kpark@knu.ac.kr
Copyright © 2014 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Fibroblast growth factor 21 (FGF21) is an attractive target for treating metabolic disease due to its wide-ranging beneficial effects on glucose and lipid metabolism. Circulating FGF21 levels are increased in insulin-resistant states; however, endogenous FGF21 fails to improve glucose and lipid metabolism in obesity, suggesting that metabolic syndrome is an FGF21-resistant state. Therefore, transcription factors for FGF21 are potential drug targets that could increase FGF21 expression in obesity and reduce FGF21 resistance. Despite many studies on the metabolic effects of FGF21, the transcriptional regulation of FGF21 gene expression remains controversial and is not fully understood. As the FGF21 transcription factor pathway is one of the most promising targets for the treatment of metabolic syndrome, further investigation of FGF21 transcriptional regulation is required.
- The fibroblast growth factor 21 (FGF21) family includes 22 members that are divided into seven subfamilies based on phylogeny and sequence [1,2]. Most FGF family members bind to FGF receptors on the cell surface and require heparin sulfate to stabilize the binding [3]. While classic FGFs act through an autocrine or paracrine mechanism to regulate cell growth and differentiation, FGF19 subfamily members including FGF15/19, FGF21, and FGF23 lack heparin-binding properties and can; therefore, be released into the circulation to act as endocrine factors [4]. In place of heparin, the transmembrane protein Klotho is required for FGF19 subfamily members to activate FGF receptors [5]; α-Klotho serves as a coreceptor for FGF23, while β-Klotho serves as a coreceptor for FGF15/19 and FGF21 [6,7,8,9].
- FGF21 is a metabolic regulator that has favorable metabolic effects on glucose and lipid metabolism [10,11,12]. As mentioned above, interaction with β-Klotho is an essential step in FGF21-receptor complex activation. β-Klotho is almost exclusively expressed in the liver, adipose tissue, and pancreas [5], which may explain why these specific tissues are the predominant site of FGF21 action, although almost all tissues express FGF receptors [13]. FGF21 has attracted attention since Kharitonenkov et al. [10] discovered its potent insulin-sensitizing actions through increased glucose uptake in rodents. It is particularly important to distinguish between the systemic pharmacological effects of FGF21 during obesity and comorbid conditions and the tissue-specific effects of FGF21 that occur under more physiological conditions (Fig. 1). The physiological actions of FGF21 occur at lower concentrations and in more restricted organ systems and tissues than the pharmacological actions [14]. In fasting, FGF21 expression is induced by peroxisome proliferator-activated receptor α (PPARα) in the liver [15,16] and acts through endocrine mechanisms for adaptive starvation responses including gluconeogenesis, ketogenesis, torpor, and inhibition of somatic growth [12,14,17]. In the fed state, FGF21 expression is induced by PPARγ in white adipose tissue and acts in an autocrine or paracrine fashion to increase PPARγ activity [14,18,19,20]. As a consequence, during feeding, the induction of FGF21 in white adipose tissue fails to increase circulating levels of FGF21 [21]. Pharmacological administration of FGF21 affects multiple organs and tissues including the pancreas, adipose tissue, liver, and the central nervous system [14]. Systemic administration of FGF21 increases insulin sensitivity and energy expenditure, causing a loss of body weight and improvements in glucose and lipid metabolism in obese rodents and primates [10,11,22]. Furthermore, several reports have also shown that administration of FGF21 results in a significant decrease in lipid accumulation in the liver of diet-induced obese mice [22], suggesting that FGF21 could be a promising drug candidate for treatment of metabolic syndrome, with benefits for many of the symptoms of this disease.
INTRODUCTION
- Serum FGF21 levels and hepatic FGF21 expression increase during obesity or type 2 diabetes [23]. However, although endogenous FGF21 fails to improve glucose and lipid metabolism during obesity [24], pharmacological administration of FGF21 improves not only insulin sensitivity but also β-cell function, which contributes to the beneficial glycemic actions of FGF21 [25]. FGF21 treatment increases islet cell number and insulin staining in db/db mice, demonstrating the ability of FGF21 to preserve β-cell mass and function [25]. Several lines of evidence suggest that FGF21 protects pancreatic β-cells by reducing β-cell glucolipotoxicity and directly reducing β-cell apoptosis via the Akt pathway [25,26,27]. As progressive β-cell loss is important for the pathophysiology of type 2 diabetes, these data suggest that FGF21 could be used to prevent the progression of type 2 diabetes.
FGF21 AND DIABETES MELLITUS
- In 2007, three groups found that fasting induces hepatic FGF21 expression and that PPARα is required for this normal starvation response [15,16,28]. PPARα, a nuclear receptor highly expressed in liver, binds directly to the FGF21 gene promoter to induce its transcription [16]. PPARα activation promotes fatty acid oxidation, ketogenesis, and gluconeogenesis [12,29]. PPARα knockout mice accumulate hepatic triglycerides and become hypoketonemic and hypoglycemic during fasting [30]. Hepatocytes are the primary source of circulating FGF21, and its synthesis is driven by the action of PPARα [16]. PPARγ is a nuclear receptor that regulates many genes involved in adipocyte differentiation, lipid synthesis and storage, insulin signaling, and glucose metabolism [31,32]. Activation of PPARγ also increases the secretion of adipokines implicated in whole-body insulin sensitization [33]. Recent studies demonstrated that PPARγ activation increases FGF21 production in adipose tissue, the secondary source of FGF21, which then acts as an autocrine or endocrine factor to improve insulin action [21,34].
TRANSCRIPTIONAL REGULATION OF FGF21 VIA UPREGULATION OF PPARα OR PPARγ
- In addition to PPARα and PPARγ, several studies have suggested that other transcription factors are involved in the regulation of hepatic FGF21 expression. Adams et al. [35]. report that thyroid hormone receptor β, which mediates the action of tri-iodothyronine in the liver, stimulates lipolysis, and hepatic fatty acid oxidation via FGF21 induction. The beneficial metabolic effects of all-trans retinoic acid (RA), which is mediated by RA receptor β (RARβ) binding, are similar to those induced by FGF21, including body weight loss and improvements in glucose and lipid metabolism [36,37]. Several researchers have speculated that FGF21 expression might be regulated by RARβ. FGF21 was characterized as a novel target gene of RARβ in hepatocytes, and hepatic RARβ can bind to putative RA responsive elements in the FGF21 promoter in a fasting-induced manner [38]. RA receptor-related orphan receptor α (RORα) is a nuclear receptor that plays a critical role in lipid metabolism [39], possibly by modulation of FGF21 secretion [40]. A recent study demonstrated that RORα directly regulates the expression and secretion of FGF21 [40]. Cyclic AMP response element-binding protein H (CREBH), an endoplasmic reticulum membrane-bound transcription factor, also induces hepatic FGF21 expression [41,42]. CREBH-deficient mice exhibit impaired fasting-induced expression of FGF21 [43]. Furthermore, CREBH is induced by fenofibrate, which is a well-known PPARα activator. We reported that CREBH mediates fenofibrate-induced suppression of hepatic lipogenesis [44]. Nur77, also known as nuclear receptor subfamily 4 group A member 1 (NR4A1), is a transcription factor in the Nur nuclear hormone receptor superfamily [45]. Nur77 expression is highly induced in adipose tissue, skeletal muscle, and liver by diverse stimuli including β-adrenergic agonists, cold exposure, and fatty acids [46,47,48,49,50,51]. Hepatic Nur77 expression is potently induced by glucagon secretion and fasting, and Nur77 is implicated in hepatic gluconeogenesis [52]. Recently, we found that during fasting, Nur77 mediates hepatic FGF21 expression and that alpha lipoic acid (ALA) increases hepatic FGF21 expression via upregulation of Nur77 (unpublished data).
REGULATION OF FGF21 EXPRESSION BY OTHER TRANSCRIPTION FACTORS
- Increased serum FGF21 concentrations are associated with obesity and insulin resistance in rodents that respond poorly to endogenous FGF21, indicating that metabolic syndrome could be the result of an FGF21-resistant state [22,24]. However, pharmacological administration of FGF21 improves glucose and lipid metabolism in diabetic rhesus monkeys, which present with the same characteristics as diabetic humans [11]. Therefore, regulators of FGF21 transcription, especially in obesity, may be potential drug targets useful for reducing FGF21 resistance. Fenofibrate, a PPARα agonist, is used clinically for the treatment of hypertriglyceridemia [53] and increases FGF21 expression via PPARα activation [54]. As mentioned previously, FGF21 is a downstream target of PPARγ, and the therapeutic effects of PPARα agonists may be mediated by stimulation of hepatic FGF21 production [16]. Thiazolidinediones, a class of antidiabetic drugs (insulin sensitizers), are well-known PPARγ agonists, and FGF21 expression can be regulated by PPARγ agonists in adipose tissue [55]. Cotreatment with FGF21 and a PPARγ agonist results in synergistic adipocyte differentiation and glucose uptake in adipose tissue [19]. Moreover, thiazolidinedione increases FGF21-induced tyrosine phosphorylation of the FGF receptor and induces β-Klotho expression [56,57]. ALA, a naturally occurring thiol antioxidant, is an essential cofactor for mitochondrial respiration [58] and is often used to manage diabetic complications [59,60]. ALA mediates a diverse range of activities including regulation of glucose and lipid metabolism by modulation of PPAR-regulated genes and key enzymes [61]. We previously demonstrated that ALA activates adenosine monophosphate-activated protein kinase and reduces lipid accumulation in livers of rodents fed a high-fat diet [62]. We also reported that ALA enhances Nur77 expression in vascular cells [63] suggesting that ALA treatment may induce nutritionally regulated gene expression in the liver through the upregulation of fasting-induced transcription factors. Recently, we found that ALA increases hepatic FGF21 expression via upregulation of Nur77 and CREBH (unpublished data). Despite many studies on FGF21, its mechanism of action remains controversial and is not fully understood. It is now clear that the FGF21 transcription factor pathway is one of the most promising drug targets for the treatment of metabolic syndrome. Therefore, further studies focused on the transcriptional regulation of FGF21 are necessary.
REGULATION OF FGF21 TRANSCRIPTION BY THERAPEUTIC AGENTS
- FGF21 has emerged as an important hormonal regulator of glucose and lipid metabolism and a promising agent for the treatment of obesity and type 2 diabetes. Therefore, it is necessary to understand the mechanisms responsible for FGF21 expression and identify other transcription factors including nuclear receptors which are able to regulate hepatic FGF21 expression.
CONCLUSIONS
-
Acknowledgements
- This work was supported by grants from the National Research Foundation of Korea (no. 2012R1A2A2A01043867) funded by the Ministry of Science, ICT & Future Planning.
ACKNOWLEDGMENTS
- 1. Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet 2004;20:563–569. ArticlePubMed
- 2. McKeehan WL, Wang F, Kan M. The heparan sulfate-fibroblast growth factor family: diversity of structure and function. Prog Nucleic Acid Res Mol Biol 1998;59:135–176. ArticlePubMed
- 3. Galzie Z, Kinsella AR, Smith JA. Fibroblast growth factors and their receptors. Biochem Cell Biol 1997;75:669–685. ArticlePubMed
- 4. Dostalova I, Haluzikova D, Haluzik M. Fibroblast growth factor 21: a novel metabolic regulator with potential therapeutic properties in obesity/type 2 diabetes mellitus. Physiol Res 2009;58:1–7. PubMed
- 5. Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S, Imamura T. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol 2008;22:1006–1014. ArticlePubMedPMCPDF
- 6. Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 2006;281:6120–6123. ArticlePubMedPMC
- 7. Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 2007;282:26687–26695. ArticlePubMedPMC
- 8. Wu X, Ge H, Gupte J, Weiszmann J, Shimamoto G, Stevens J, Hawkins N, Lemon B, Shen W, Xu J, Veniant MM, Li YS, Lindberg R, Chen JL, Tian H, Li Y. Co-receptor requirements for fibroblast growth factor-19 signaling. J Biol Chem 2007;282:29069–29072. ArticlePubMed
- 9. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006;444:770–774. ArticlePubMedPDF
- 10. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest 2005;115:1627–1635. ArticlePubMedPMC
- 11. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007;148:774–781. ArticlePubMedPDF
- 12. Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 2008;8:77–83. ArticlePubMedPMC
- 13. Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, Kharitonenkov A. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab 2012;2:31–37. ArticlePubMedPMC
- 14. Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev 2012;26:312–324. ArticlePubMedPMC
- 15. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007;5:426–437. ArticlePubMed
- 16. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007;5:415–425. ArticlePubMed
- 17. Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature 2006;439:340–343. ArticlePubMedPDF
- 18. Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem 2006;281:28721–28730. ArticlePubMed
- 19. Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol 2008;74:403–412. ArticlePubMed
- 20. Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol 2008;28:188–200. ArticlePubMed
- 21. Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell 2012;148:556–567. ArticlePubMedPMC
- 22. Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008;149:6018–6027. ArticlePubMedPDF
- 23. Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008;57:1246–1253. ArticlePubMed
- 24. Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010;59:2781–2789. ArticlePubMedPMC
- 25. Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 2006;55:2470–2478. ArticlePubMed
- 26. Woo YC, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin Endocrinol (Oxf) 2013;78:489–496. ArticlePubMed
- 27. Kliewer SA, Mangelsdorf DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr 2010;91:254S–257S. ArticlePubMedPDF
- 28. Lundasen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun 2007;360:437–440. ArticlePubMed
- 29. Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest 1999;103:1489–1498. ArticlePubMedPMC
- 30. Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK, Rao MS. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem 2000;275:28918–28928. ArticlePubMed
- 31. Fajas L, Debril MB, Auwerx J. PPAR gamma: an essential role in metabolic control. Nutr Metab Cardiovasc Dis 2001;11:64–69.
- 32. Lehrke M, Lazar MA. The many faces of PPARgamma. Cell 2005;123:993–999. ArticlePubMed
- 33. Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 2006;281:2654–2660. ArticlePubMed
- 34. Drakulic BJ, Juranic IO, Eric S, Zloh M. Role of complexes formation between drugs and penetration enhancers in transdermal delivery. Int J Pharm 2008;363:40–49. ArticlePubMed
- 35. Adams AC, Astapova I, Fisher FM, Badman MK, Kurgansky KE, Flier JS, Hollenberg AN, Maratos-Flier E. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPARalpha-dependent manner. J Biol Chem 2010;285:14078–14082. ArticlePubMedPMC
- 36. Berry DC, DeSantis D, Soltanian H, Croniger CM, Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes 2012;61:1112–1121. ArticlePubMedPMC
- 37. Tsuchiya H, Ikeda Y, Ebata Y, Kojima C, Katsuma R, Tsuruyama T, Sakabe T, Shomori K, Komeda N, Oshiro S, Okamoto H, Takubo K, Hama S, Shudo K, Kogure K, Shiota G. Retinoids ameliorate insulin resistance in a leptin-dependent manner in mice. Hepatology 2012;56:1319–1330. ArticlePubMed
- 38. Li Y, Wong K, Walsh K, Gao B, Zang M. Retinoic acid receptor beta stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole-body energy homeostasis in mice. J Biol Chem 2013;288:10490–10504. ArticlePubMedPMC
- 39. Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, Russell LB, Mueller KL, van Berkel V, Birren BW, Kruglyak L, Lander ES. Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature 1996;379:736–739. ArticlePubMedPDF
- 40. Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J Biol Chem 2010;285:15668–15673. ArticlePubMedPMC
- 41. Chin KT, Zhou HJ, Wong CM, Lee JM, Chan CP, Qiang BQ, Yuan JG, Ng IO, Jin DY. The liver-enriched transcription factor CREB-H is a growth suppressor protein underexpressed in hepatocellular carcinoma. Nucleic Acids Res 2005;33:1859–1873. ArticlePubMedPMCPDF
- 42. Omori Y, Imai J, Watanabe M, Komatsu T, Suzuki Y, Kataoka K, Watanabe S, Tanigami A, Sugano S. CREB-H: a novel mammalian transcription factor belonging to the CREB/ATF family and functioning via the box-B element with a liver-specific expression. Nucleic Acids Res 2001;29:2154–2162. ArticlePubMedPMCPDF
- 43. Lee JH, Giannikopoulos P, Duncan SA, Wang J, Johansen CT, Brown JD, Plutzky J, Hegele RA, Glimcher LH, Lee AH. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nat Med 2011;17:812–815. ArticlePubMedPMCPDF
- 44. Min AK, Jeong JY, Go Y, Choi YK, Kim YD, Lee IK, Park KG. cAMP response element binding protein H mediates fenofibrate-induced suppression of hepatic lipogenesis. Diabetologia 2013;56:412–422. ArticlePubMedPDF
- 45. Chang C, Kokontis J, Liao SS, Chang Y. Isolation and characterization of human TR3 receptor: a member of steroid receptor superfamily. J Steroid Biochem 1989;34:391–395. ArticlePubMed
- 46. Ohkura N, Ito M, Tsukada T, Sasaki K, Yamaguchi K, Miki K. Structure, mapping and expression of a human NOR-1 gene, the third member of the Nur77/NGFI-B family. Biochim Biophys Acta 1996;1308:205–214. ArticlePubMed
- 47. Ohkura N, Hijikuro M, Yamamoto A, Miki K. Molecular cloning of a novel thyroid/steroid receptor superfamily gene from cultured rat neuronal cells. Biochem Biophys Res Commun 1994;205:1959–1965. ArticlePubMed
- 48. Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 2006;126:789–799. ArticlePubMedPMC
- 49. Lim RW, Yang WL, Yu H. Signal-transduction-pathway-specific desensitization of expression of orphan nuclear receptor TIS1. Biochem J 1995;308(Pt 3):785–789. ArticlePubMedPMCPDF
- 50. Ohkura N, Ito M, Tsukada T, Sasaki K, Yamaguchi K, Miki K. Alternative splicing generates isoforms of human neuron-derived orphan receptor-1 (NOR-1) mRNA. Gene 1998;211:79–85. ArticlePubMed
- 51. Maltais A, Labelle Y. Structure and expression of the mouse gene encoding the orphan nuclear receptor TEC. DNA Cell Biol 2000;19:121–130. ArticlePubMed
- 52. Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med 2006;12:1048–1055. ArticlePubMedPDF
- 53. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A 1997;94:4312–4317. ArticlePubMedPMC
- 54. Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008;8:169–174. ArticlePubMed
- 55. Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 1997;272:3406–3410. ArticlePubMed
- 56. Moyers JS, Shiyanova TL, Mehrbod F, Dunbar JD, Noblitt TW, Otto KA, Reifel-Miller A, Kharitonenkov A. Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARgamma signaling. J Cell Physiol 2007;210:1–6. ArticlePubMed
- 57. Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, Hale JE, Coskun T, Shanafelt AB. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol 2008;215:1–7. ArticlePubMed
- 58. Lee GH, Bhandary B, Lee EM, Park JK, Jeong KS, Kim IK, Kim HR, Chae HJ. The roles of ER stress and P450 2E1 in CCl(4)-induced steatosis. Int J Biochem Cell Biol 2011;43:1469–1482. ArticlePubMed
- 59. Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schutte K, Gries FA. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995;38:1425–1433. ArticlePubMedPDF
- 60. Ziegler D, Gries FA. Alpha-lipoic acid in the treatment of diabetic peripheral and cardiac autonomic neuropathy. Diabetes 1997;46(Suppl 2):S62–S66. ArticlePubMed
- 61. McCarty MF, Barroso-Aranda J, Contreras F. The "rejuvenatory" impact of lipoic acid on mitochondrial function in aging rats may reflect induction and activation of PPAR-gamma coactivator-1alpha. Med Hypotheses 2009;72:29–33. ArticlePubMed
- 62. Park KG, Min AK, Koh EH, Kim HS, Kim MO, Park HS, Kim YD, Yoon TS, Jang BK, Hwang JS, Kim JB, Choi HS, Park JY, Lee IK, Lee KU. Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology 2008;48:1477–1486. ArticlePubMed
- 63. Kim HJ, Kim JY, Lee SJ, Oh CJ, Choi YK, Lee HJ, Do JY, Kim SY, Kwon TK, Choi HS, Lee MO, Park IS, Park KG, Lee KU, Lee IK. Alpha-lipoic acid prevents neointimal hyperplasia via induction of p38 mitogen-activated protein kinase/Nur77-mediated apoptosis of vascular smooth muscle cells and accelerates postinjury reendothelialization. Arterioscler Thromb Vasc Biol 2010;30:2164–2172. ArticlePubMed
References
Fig. 1Endocrine, autocrine, and pharmacological actions of fibroblast growth factor 21 (FGF21) and its transcription factors. Fasting induces FGF21 expression in the liver through several transcription factors, and FGF21 acts as an endocrine hormone to induce ketogenesis, gluconeogenesis, fatty acid oxidation, and torpor and to inhibit somatic growth. In the fed state, FGF21 expression in white adipose tissue is induced by peroxisome proliferator-activated receptor gamma (PPARγ), and FGF21 acts through an autocrine or paracrine mechanism to increase PPARγ activity. Pharmacological administration of recombinant FGF21 (rFGF21) affects multiple tissues and has beneficial effects on lipid and glucose metabolism in metabolic disease, including obesity and diabetes mellitus. Recent studies have demonstrated that several metabolically-active drugs produce hepatic FGF21, suggesting a relationship between their actions in glucose and lipid metabolism with the up-regulation of FGF21 production. CREBH, cyclic adenosine monophosphate (AMP) response element-binding protein H; RARβ, retinoic acid (RA) receptor β; RORα, RA receptor-related orphan receptor α; Nur77, nerve growth factor IB; TRβ, thyroid hormone receptor β; T2DM, type 2 diabetes mellitus.
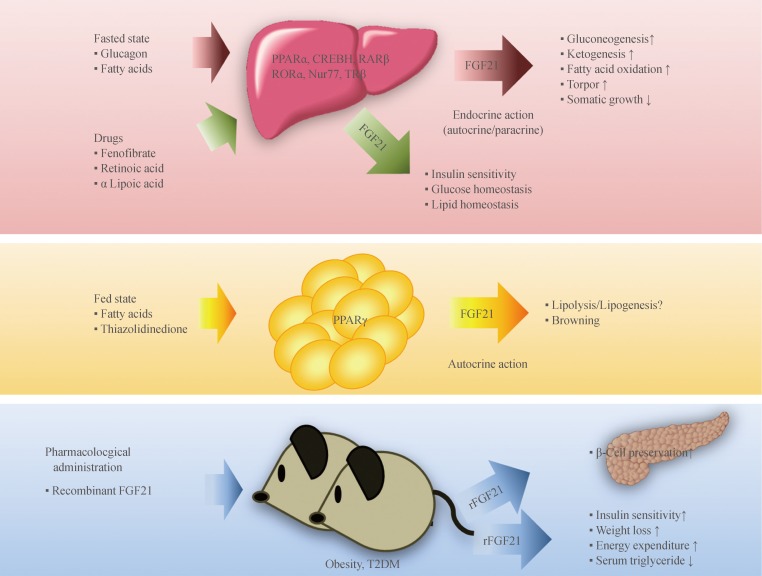

Figure & Data
References
Citations
Citations to this article as recorded by 

- Glucosamine Enhancement of Learning and Memory Functions by Promoting Fibroblast Growth Factor 21 Production
Yu-Ming Chao, Hon-Yen Wu, Sin-Huei Yeh, Ding-I Yang, Lu-Shiun Her, Yuh-Lin Wu
International Journal of Molecular Sciences.2024; 25(8): 4211. CrossRef - Relationship between FGF21 and drug or nondrug therapy of type 2 diabetes mellitus
Chang Guo, Li Zhao, Yanyan Li, Xia Deng, Guoyue Yuan
Journal of Cellular Physiology.2021; 236(1): 55. CrossRef - Serum fibroblast growth factor 21 levels after out of hospital cardiac arrest are associated with neurological outcome
Pirkka T. Pekkarinen, Markus B. Skrifvars, Ville Lievonen, Pekka Jakkula, Laura Albrecht, Pekka Loisa, Marjaana Tiainen, Ville Pettilä, Matti Reinikainen, Johanna Hästbacka
Scientific Reports.2021;[Epub] CrossRef - Epigenetic Regulation of Processes Related to High Level of Fibroblast Growth Factor 21 in Obese Subjects
Teresa Płatek, Anna Polus, Joanna Góralska, Urszula Raźny, Agnieszka Dziewońska, Agnieszka Micek, Aldona Dembińska-Kieć, Bogdan Solnica, Małgorzata Malczewska-Malec
Genes.2021; 12(2): 307. CrossRef - Nutritional Regulation of Hepatic FGF21 by Dietary Restriction of Methionine
Han Fang, Kirsten P. Stone, Laura A. Forney, Desiree Wanders, Thomas W. Gettys
Frontiers in Endocrinology.2021;[Epub] CrossRef - The Presence of Urinary Ketones according to Metabolic Status and Obesity
Bo-Reum Kim, Jeong Woo Seo, Sang Man Kim, Kyu-Nam Kim, Nam-Seok Joo
Journal of Korean Medical Science.2020;[Epub] CrossRef - MS-275 induces hepatic FGF21 expression via H3K18ac-mediated CREBH signal
Qi Zhang, Qin Zhu, Ruyuan Deng, Feiye Zhou, Linlin Zhang, Shushu Wang, Kecheng Zhu, Xiao Wang, Libin Zhou, Qing Su
Journal of Molecular Endocrinology.2019; 62(4): 187. CrossRef - Spontaneous ketonuria and risk of incident diabetes: a 12 year prospective study
Gyuri Kim, Sang-Guk Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Ele Ferrannini, Yong-ho Lee, Nam H. Cho
Diabetologia.2019; 62(5): 779. CrossRef - Fibroblast Growth Factor 21 and the Adaptive Response to Nutritional Challenges
Úrsula Martínez-Garza, Daniel Torres-Oteros, Alex Yarritu-Gallego, Pedro F. Marrero, Diego Haro, Joana Relat
International Journal of Molecular Sciences.2019; 20(19): 4692. CrossRef - Berberine-induced activation of AMPK increases hepatic FGF21 expression via NUR77
Feiye Zhou, Mengyao Bai, Yuqing Zhang, Qin Zhu, Linlin Zhang, Qi Zhang, Shushu Wang, Kecheng Zhu, Yun Liu, Xiao Wang, Libin Zhou
Biochemical and Biophysical Research Communications.2018; 495(2): 1936. CrossRef - Practical prospects for boosting hepatic production of the “pro-longevity” hormone FGF21
Mark F. McCarty
Hormone Molecular Biology and Clinical Investigation.2017;[Epub] CrossRef - The regulation of FGF21 gene expression by metabolic factors and nutrients
Anjeza Erickson, Régis Moreau
Hormone Molecular Biology and Clinical Investigation.2017;[Epub] CrossRef - Diabetes Mellitus and Sepsis
Silvia C. Trevelin, Daniela Carlos, Matteo Beretta, João S. da Silva, Fernando Q. Cunha
Shock.2017; 47(3): 276. CrossRef - The U-shaped relationship between fibroblast growth factor 21 and microvascular complication in type 2 diabetes mellitus
Chan-Hee Jung, Sang-Hee Jung, Bo-Yeon Kim, Chul-Hee Kim, Sung-Koo Kang, Ji-Oh Mok
Journal of Diabetes and its Complications.2017; 31(1): 134. CrossRef - Fibroblast Growth Factor 21—Metabolic Role in Mice and Men
Harald Staiger, Michaela Keuper, Lucia Berti, Martin Hrabě de Angelis, Hans-Ulrich Häring
Endocrine Reviews.2017; 38(5): 468. CrossRef - Anti-inflammatory effects of exercise training in adipose tissue do not require FGF21
Jay W Porter, Joe L Rowles, Justin A Fletcher, Terese M Zidon, Nathan C Winn, Leighton T McCabe, Young-Min Park, James W Perfield, John P Thyfault, R Scott Rector, Jaume Padilla, Victoria J Vieira-Potter
Journal of Endocrinology.2017; 235(2): 97. CrossRef - Hepatic Fgf21 Expression Is Repressed after Simvastatin Treatment in Mice
Panos Ziros, Zoi Zagoriti, George Lagoumintzis, Venetsana Kyriazopoulou, Ralitsa P. Iskrenova, Evagelia I. Habeos, Gerasimos P. Sykiotis, Dionysios V. Chartoumpekis, Ioannis G Habeos, Kostas Pantopoulos
PLOS ONE.2016; 11(9): e0162024. CrossRef - Association between insulin resistance and impairment of FGF21 signal transduction in skeletal muscles
Ja Young Jeon, Sung-E Choi, Eun Suk Ha, Tae Ho Kim, Jong Gab Jung, Seung Jin Han, Hae Jin Kim, Dae Jung Kim, Yup Kang, Kwan-Woo Lee
Endocrine.2016; 53(1): 97. CrossRef - Fibroblast growth factor 21 deficiency exacerbates chronic alcohol-induced hepatic steatosis and injury
Yanlong Liu, Cuiqing Zhao, Jian Xiao, Liming Liu, Min Zhang, Cuiling Wang, Guicheng Wu, Ming-Hua Zheng, Lan-Man Xu, Yong-Ping Chen, Moosa Mohammadi, Shao-Yu Chen, Matthew Cave, Craig McClain, Xiaokun Li, Wenke Feng
Scientific Reports.2016;[Epub] CrossRef - The Impact of Organokines on Insulin Resistance, Inflammation, and Atherosclerosis
Kyung Mook Choi
Endocrinology and Metabolism.2016; 31(1): 1. CrossRef - Physiological and Pharmacological Roles of FGF21 in Cardiovascular Diseases
Peng Cheng, Fangfang Zhang, Lechu Yu, Xiufei Lin, Luqing He, Xiaokun Li, Xuemian Lu, Xiaoqing Yan, Yi Tan, Chi Zhang
Journal of Diabetes Research.2016; 2016: 1. CrossRef - Minireview: Roles of Fibroblast Growth Factors 19 and 21 in Metabolic Regulation and Chronic Diseases
Fangfang Zhang, Lechu Yu, Xiufei Lin, Peng Cheng, Luqing He, Xiaokun Li, Xuemian Lu, Yi Tan, Hong Yang, Lu Cai, Chi Zhang
Molecular Endocrinology.2015; 29(10): 1400. CrossRef - AMP-activated protein kinase suppresses the expression of LXR/SREBP-1 signaling-induced ANGPTL8 in HepG2 cells
Jinmi Lee, Seok-Woo Hong, Se Eun Park, Eun-Jung Rhee, Cheol-Young Park, Ki-Won Oh, Sung-Woo Park, Won-Young Lee
Molecular and Cellular Endocrinology.2015; 414: 148. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite


